Introduction
This policy brief explains why it would be misguided and counterproductive to expand the World Trade Organization’s “Covid” waiver of the obligation to enforce patents on vaccines to also cover Covid therapeutics and diagnostics. We provide five reasons why WTO members should not expand the waiver.
The waiver to the WTO Agreement on Trade Related Aspects of IP (TRIPS Agreement), agreed by WTO members in June 2022, currently allows low- and middle-income countries to temporarily waive protections on Covid vaccine patents to allow members to produce the shots for use domestically or shipment abroad. The WTO is now considering whether to expand the Covid waiver to therapeutics and tests, a decision due by December 2022.
Expanding the waiver would be a mistake, as it would:
- Derail current successful voluntary efforts to expand access to Covid treatments;
- Fail to address the challenges of health systems and other institutions that are undermining access to existing treatments;
- Jeopardise innovation incentives for diseases unrelated to Covid;
- Enable certain WTO members to gain industrial advantage at the expense of other countries; and
- Destroy incentives to develop new treatments for Covid-19 and indeed future pandemics.

The way forward
Many of the solutions to insufficient access to Covid therapeutics are the same as those for vaccines:
- The trade dimension remains important, with trade barriers at and behind the border a major factor behind delayed global distribution of therapeutic drugs. WTO members should also extend tariff exemptions to Covid medicines, and refrain from imposing export restrictions.
- Improving in-country readiness and testing infrastructure, in collaboration with international partners
- Funding and political commitment to multilateral Covid organisations
- Maintaining existing levels of IP protection, to promote R&D and manufacturing.
The waiver expansion does not address any of the main barriers to Covid therapeutic access. It would undermine current and future R&D and derail existing manufacturing partnerships. Its negative effects would likely spill over into other therapeutic areas, undermining innovation globally while promoting narrow industrial interests of a minority of WTO members.
As with the original TRIPS waiver, any expansion is unnecessary, counterproductive and wrong.
1. Innovators are already voluntarily collaborating with generic companies to produce covid therapeutics, but a waiver expansion could derail these efforts
Throughout the pandemic, innovators have collaborated and shared technology with hundreds of partners around the world to deliver Covid vaccines and treatments to billions of people. Against the expectations of many, IP rights enabled this cooperation rather than impeding it. The surprise many expressed at these collaborations (and, in some cases, continuing and stubborn denial of the facts) is based on a misunderstanding of the role of IP rights.
IP rights support collaboration by providing security to innovators. With legal protection, they can teach manufacturing partners – even their biggest competitors – how to make their products without fear that they are giving away their hard-earned and costly research successes.
This IP-driven collaboration is exactly what happened during the pandemic. We have previously documented this collaborative success in battling Covid-19 through first-hand accounts and in-depth research. We found that trade secret protection was particularly important, enabling innovators to share documentation of sensitive know-how and provide teams of employees to teach manufacturing techniques.
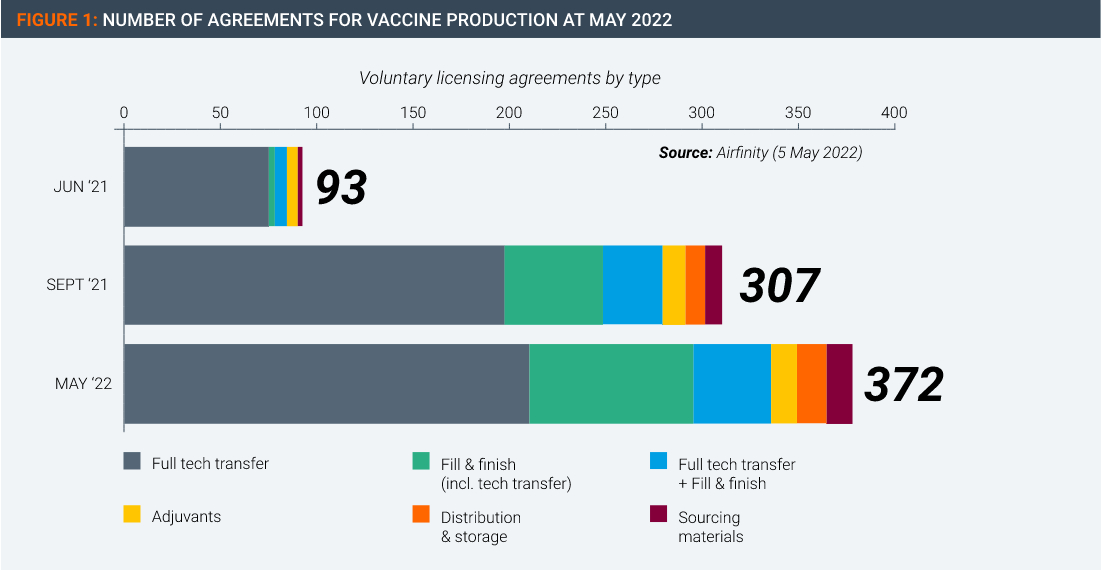
Thanks to the hundreds of voluntary licensing agreements owners of vaccine technologies have signed and implemented with other manufacturers throughout the world (Figure 1), the world now has an oversupply of vaccines.
Innovators have also been successfully collaborating with partners to manufacture Covid therapeutics, most of which have been developed later than vaccines.
For example, in May 2020, Gilead launched a royalty-free voluntary licensing program for Remdesivir (Veklury), working with nine generic pharmaceutical manufacturers, covering 127 countries. By July 2022, Gilead reported that the licensing program had more than 11 million people with generic doses of Remdesivir, including 7 million people in 127 low- and middle-income countries. As part of this program, Gilead has provided support to its generic partners to enable them to ramp up production.
In November 2021, Pfizer struck a royalty-free licensing deal with the Medicines Patent Pool to enable it to create a network of generic manufacturers for Pfizer’s Paxlovid (nirmatrelvir/ritonavir -NIR/r) antiviral pill. By March 2022, 35 generic companies in 12 countries had signed on to manufacture generic Paxlovid, providing access to about 53% of the world’s population across 95 low and middle-income countries. Under a recent deal announced by the Clinton Health Access Initiative, generic suppliers will make 4.5 million doses of generic Paxlovid per month for patients in low- and middle-income countries.
The availability of royalty-free licenses for generic manufacturing of Veklury and Paxlovid is significant, as these two therapeutics are currently the therapeutics recommended by experts such as the U.S. National Institute of Health as preferred treatments for Covid-19.
Other therapeutics have been made available on similar terms for royalty-free generic manufacture. For example, the Medicines Patent Pool has entered into a license agreement with Merck Sharp & Dohme (MSD), for generic manufacture of its anti-viral pill Molnupiravir for distribution into nearly 100 low and middle-income countries. These licenses also provide tech transfer support as part of the agreement to assist with quality control and regulatory submissions.
Voluntary licenses are the best way of rapidly transferring technology to partners as they allow for an orderly and safe transfer of technical manufacturing know-how, much of which needs to be taught in person. This is particularly true for more complex medical technologies such as novel Covid vaccines and novel Covid treatments, many of which are complex biological products. As MPP has stated, it vetted its sublicensees for the production of Paxlovid for “their ability to meet MPP’s requirements related to production capacity, regulatory compliance, as well as international standards for quality-assured medicines.”
Voluntary licenses allow for the transfer of technology while protecting IP rights, ensuring IP can play its key economic role of encouraging investment into future R&D. Further, voluntary agreements such as these ensure that technology can be transferred rapidly without risk of legal hold-up in the event of public health emergencies such as pandemics.

A TRIPS waiver expansion would undermine these voluntary agreements in two ways.
- First, the voluntary agreements have ensured that resources have been directed to the generic manufacturers most capable of producing generic therapeutics effectively. For example, in the case of the MPP sublicenses, MPP vetted suppliers for capability to manufacture effectively and safely. Moreover, in all the instances described here, the innovator provided technical assistance with building manufacturing capacity and regulatory compliance. In a time of supply shortages and constrained supply chains, it has been important to ensure that resources are provided to those best able to employ them. A waiver would create competition for scarce resources, diverting some to manufacturers that were less capable and lacked the support provided pursuant to voluntary licenses.
- Second, a waiver would make voluntary licensing less attractive to both innovators and generic firms. In our research for our previous report, innovators explained to us that a loss of IP rights would make them less willing to collaborate widely. The change in behaviour would not be punitive. Rather, they would need to protect investments in R&D because of responsibilities to employees, investors, and other stakeholders by limiting exposure to countries and potential competitors not committed to respecting their IP rights.
A waiver could also weaken the position of the generic manufacturers who have in good faith entered into licensing arrangements, investing in systems and facilities to ensure their products meet the highest regulatory standards. Most of these generic manufacturers are based in middle- and low-income countries.
In fact, given the tremendous demand and the fact that these licenses are royalty-free, the only constraint on supply has been capability, not the exclusivity potentially enabled by IP rights. Undermining voluntary licensing would harm an essential source for the manufacture of quality-assured products of the highest standards.
2. Supplies of therapeutics have been sufficient to meet demand, but the ability of healthcare systems to deliver treatments continues to be a challenge
Advocates of the TRIPS waiver contend that the existing IP-based system creates artificial scarcity of Covid therapeutics, particularly for those countries with limited economic means. The facts belie this assertion.
First, as noted above, the most important therapeutics have been available to manufacture under royalty-free licenses for at least a year. In the case of generic Paxlovid and Molnupiravir, those licenses were vetted and administered by the Medicines Patent Pool, and large networks of generic licensees have been created. IP has not been the constraint in these instances.
Moreover, there is no evidence of an undersupply of Covid therapeutics. According to data supplied by Airfinity, global production of a range of Covid therapeutics has exceeded the amount demanded in the form of contracted-for supply thus far, in some cases by significant amounts (Figure 2).
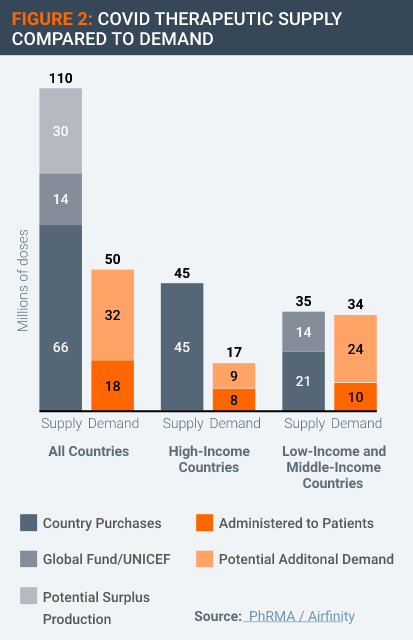 This supply and demand data could be interpreted in more than one way. For example, if prices were high and if supply were constrained by a lack of competition, these conditions could produce a lower quantity demanded.
This supply and demand data could be interpreted in more than one way. For example, if prices were high and if supply were constrained by a lack of competition, these conditions could produce a lower quantity demanded.
However, high prices and constrained competition do not reflect actual market conditions. Instead, the facts on the ground consist of royalty-free licenses, widespread participation in the licensing programs, and the presence of donor organizations purchasing large quantities from generic manufacturers for those least able to afford medicines.
In light of these conditions, if Covid therapeutics are being under-used, the problem does not stem from IP rights, prices, or constraints on competition. Instead, the likely challenge lies with difficulties faced by health systems in the delivery of treatments to patients. An IP waiver would have no positive effect on this problem.
The health systems challenges to delivering Covid therapeutics to patients are the same or similar to those that have slowed uptake of Covid-19 vaccines once global supplies became sufficient. Access to doctors and health facilities is limited in many regions. Infrastructure can make delivery of any supplies difficult. Public health systems often lack resources. Taken together, these kinds of issues prevent treatments getting to patients even when supplies are plentiful.
The difficulty in delivering Covid therapeutics was explored in a recent article in Nature entitled “COVID antibody drugs have saved lives — so why aren’t they more popular?” The specific type of therapeutic the article addressed – monoclonal antibodies – must be delivered by IV infusion. As Nature explained, “Health-care systems have struggled to distribute COVID-19 antibodies effectively and equitably, even more so than they did with vaccines and antiviral medicines such as Paxlovid. Not only do these drugs need to be given early in the course of infection for best effect, but the first COVID-19 antibodies were also best delivered by intravenous drip. This created diagnostic, infrastructural, staffing and other bottlenecks.”
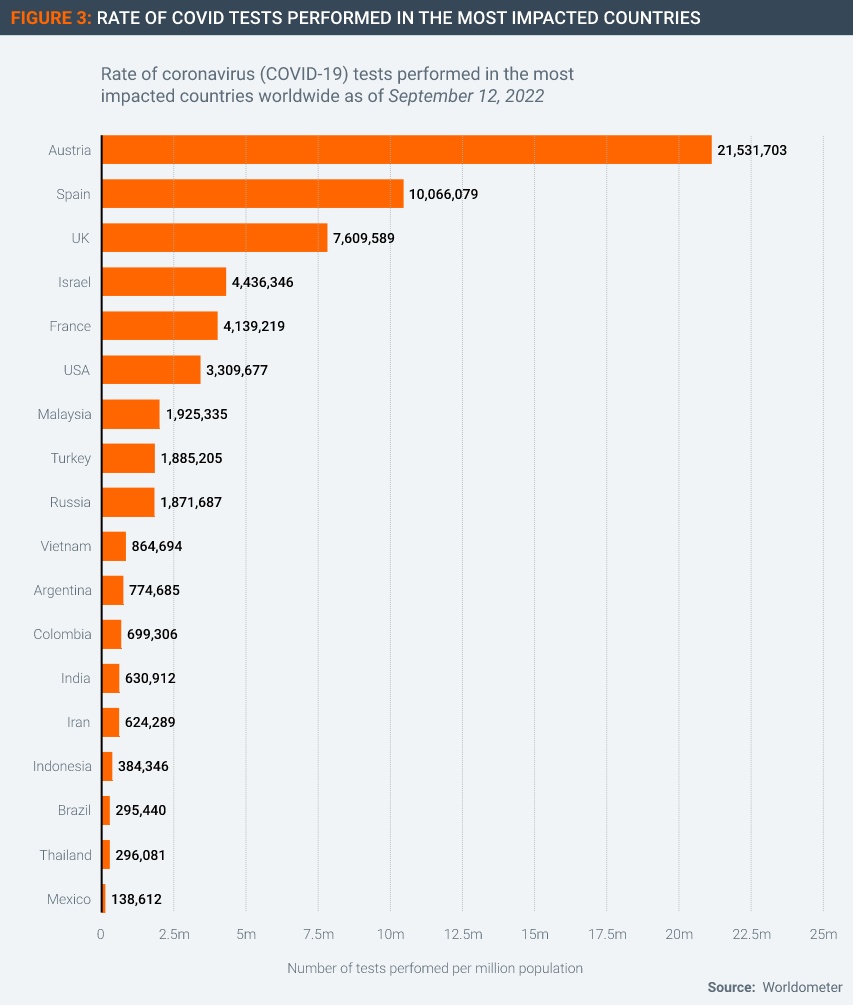
Another reason for the lack of demand for therapeutics is that some of the worst affected countries have been testing at very low rates relative to the scale of Covid infections (Figure 3). Without the information provided by test results, it is difficult for health authorities to plan and allocate therapeutics to those in clinical need. Testing volumes have fallen globally since the Omicron wave peaked in early 2022.
Correcting these imbalances is a matter of health systems policy, not intellectual property as envisaged with the WTO TRIPS waiver expansion.
3. An expanded waiver would jeopardise innovation incentives for diseases unrelated to covid
Several Covid treatments are drugs developed to address other disease conditions that have been repurposed to treat Covid. It is not clear how the waiver expansion text could be worded in such a way as to ensure that any medicine used to treat Covid-19 is used for that purpose and that purpose only.
As Figures 4 & 5 show, many Covid treatments either approved or under study have uses for multiple viruses or illnesses beyond Covid.
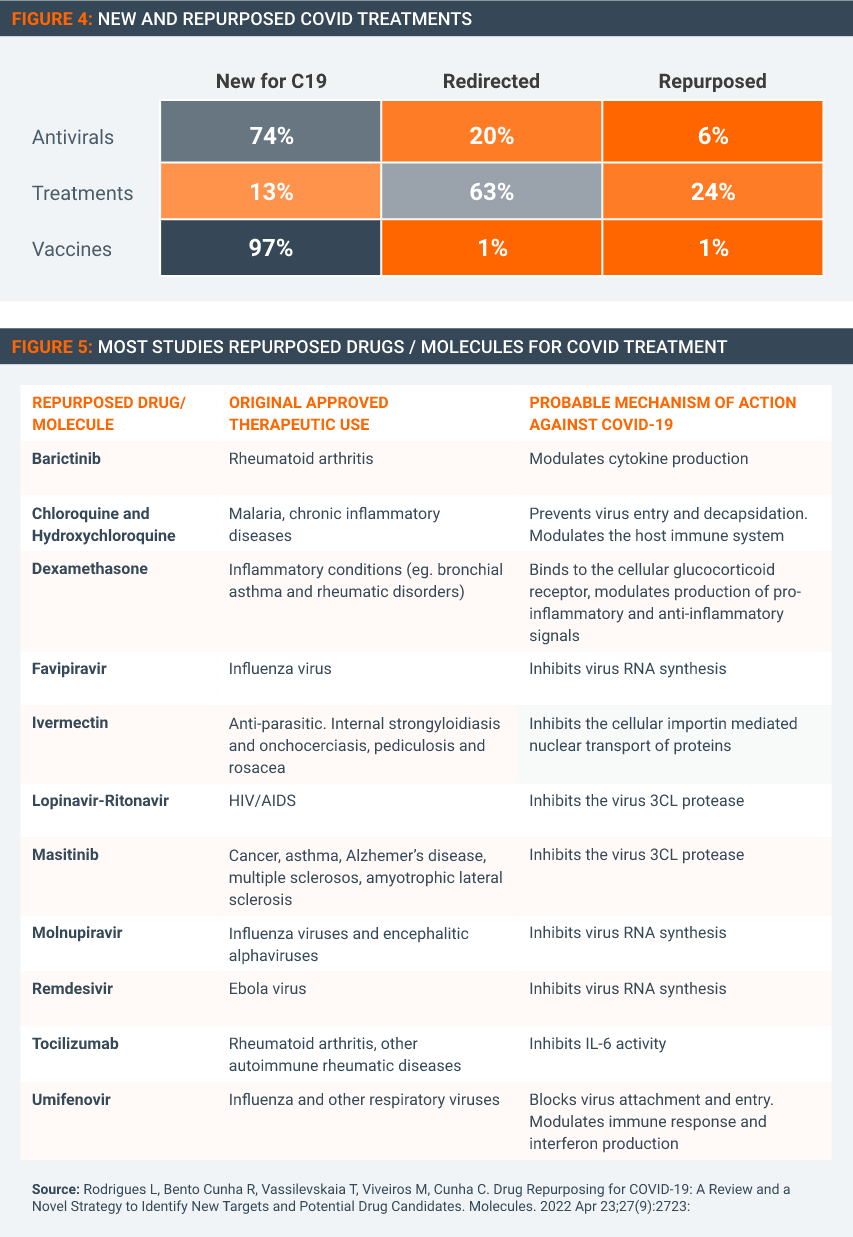
Some of the medicines repurposed or with potential for Covid-19 (such as dexamethasone) are long off-patent. But many are innovative medicines that still have patent term remaining in many countries. It would be near impossible to draft and agree to TRIPS expansion text that legally guarantees that these patented therapeutics will not be used for any other condition than Covid. Enforcement would be equally problematic. A waiver expansion to encompass therapeutics and diagnostics would then result in all kinds of medicines with applications beyond Covid becoming susceptible for compulsory licenses.
This circumstance would create counterproductive, even perverse, incentives. In several instances, drugs were repurposed for Covid after the innovator invested substantial sums in testing to determine if their existing treatment was effective against Covid. Gilead’s Remdesivir was one such example, with the company spending tens of millions of dollars in testing and regulatory compliance costs. Other innovators supplied proprietary data and doses of their medicine to researchers to assist in efforts to find candidates for repurposing.
However, if repurposing a drug results in the loss of existing markets for that drug, then such voluntary efforts will cease. An expanded waiver would create the incentive to not explore repurposing and to avoid cooperating in repurposing research. The results could be extremely harmful – and not just in a future pandemic, but in the current one as well, because research on repurposing existing drugs to treat Covid-19 is ongoing. Ultimately, patients would be the losers.

4. A waiver expansion would allow WTO members to exploit proprietary technologies for their own industrial advantage
The major inventions that have made inroads to the Covid-19 pandemic, such as the mRNA vaccine technology platform and the anti-viral Paxlovid, are novel technologies that are the result of considerable private sector R&D investment and effort. These and similar technologies have the potential for a wide range of health applications beyond Covid.
It would be difficult if not impossible to limit the expanded waiver to specific countries. Further, it would be impossible in practice to enforce the use of Covid technologies to Covid only, across all WTO members.
A major function of the WTO TRIPS Agreement is to create a global level playing field of enforceable IP rules, allowing companies and countries to complete equally in the production and trade of knowledge-based goods and services. Creating exemptions of IP-rules for potentially broadly-applicable categories of technologies fundamentally undermines the raison d’être of the TRIPS Agreement, raising the real possibility countries could exploit the waiver expansion to further their own industrial policy goals.
Some suggest that China is not able to avail itself of the new flexibilities in the TRIPS waiver but there is little legal basis to enforce that. China could then issue compulsory licenses under the TRIPS waiver to gain access to new proprietary technologies currently approved for use against Covid. It would gain valuable experience in working with these technologies, and there would be little legal recourse to prevent companies or entities there from re-orienting the technologies to disease indications that have greater global potential for profit-making.
The waiver expansion runs the risk of turning the TRIPS Agreement into the inverse of its true purpose, a vehicle for using the proprietary technologies of others for self-interested industrial policy purposes.
5. A waiver expansion could severely harm current and future covid therapeutic R&D
The existing IP system is presiding over a huge level of research and development activity into Covid-19 therapeutics. According to BIO, there were 205 antivirals and 305 treatments under development as of 19th September 2022.
These R&D projects encompass a range of public-private partnerships and in-house programmes that would become far less viable in light of an expanded IP waiver. At least some of this research will likely cease if the TRIPS waiver is expanded, as investments become less secure and potential returns evaporate. Governments alone could not step up to fill these R&D gaps, given the amounts of capital and skill required.
The future pipeline for Covid therapeutics would be constrained or might even dry up. In the event of a future pandemic, the precedent would make private sector companies less likely to involve themselves R&D if the end result is confiscation. Their investors and stockholders would likely respond by finding safer investments, an intolerable and counterproductive result.
The concern is not theoretical, as experience has shown that innovators adjust to past policy changes. A 2018 report related that innovators had felt “burned” in past pandemics when governments reneged on promises to purchase vaccines after those pandemics turned out to be less dangerous than feared. As a result, innovators at that time were curtailing vaccine research programs and taking a more conservative approach.
As the article explained, “nearly all the major pharmaceutical companies that work on these vaccines have found themselves holding the bag after at least one of these outbreaks.” They lost significant sums in the case of the H1N1 virus, after governments reneged on commitments to purchase vaccines. The result of this scepticism was that governments needed to make much more solid and substantial advanced purchase commitments for vaccines at the start of the Covid-19 pandemic.
Losing investments in therapeutics would have a similar and further effect. Imposing costs for engaging in the most socially beneficial research will result in less socially beneficial research in the future.
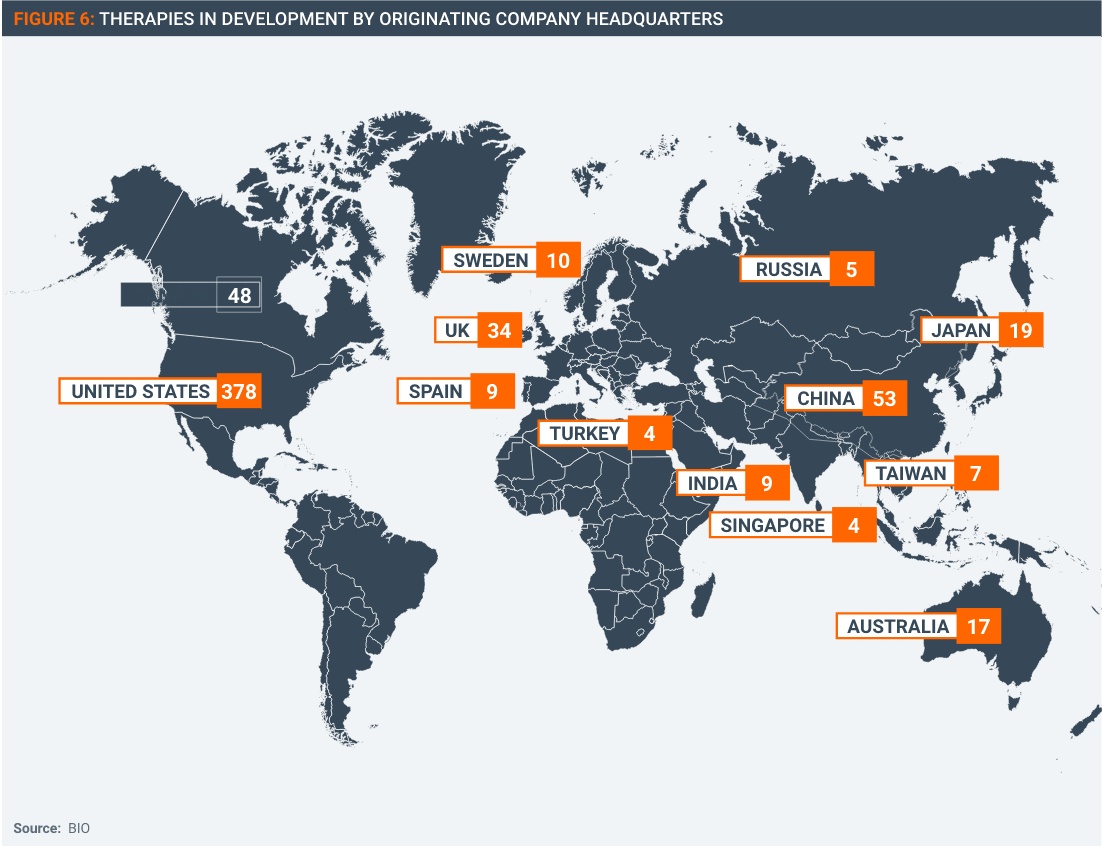
This not an issue confined to manufacturers in the United States and Europe: companies and entities from all over the world would be impacted (Figure 6).