With careful reform of its patent law Indonesia can accelerate its transition to a knowledge-based, wealthy economy
As the Indonesian economy recovers from Covid, the government is looking to target new sources of economic growth to counter the country’s reliance on natural resources, avoid the middle-income trap and become a high-income economy.
Economists generally agree that sustainable economic growth depends on higher value services, manufacturing, research and development (R&D), and less reliance on commodities and natural resources. Companies’ increasing desire to diversify high-tech manufacturing supply chains presents a great opportunity.
Indonesia’s domestic manufacturing sector is focused on the low-value assembly of products designed and manufactured elsewhere. It generates low-quality jobs and little economic value or tax revenue. Export of commodities such as oil and gas is prone to cyclical fluctuation and does not present a long term solution given the global transition to zero carbon.
For Indonesia to join the ranks of high-income countries in the longer term it needs to diversify beyond these sectors and further build its nascent knowledge economy. Knowledge-based industries – such as biopharmaceuticals, information technology, chemicals and entertainment – underpin sustainable growth and employment in the economies of high-income countries.
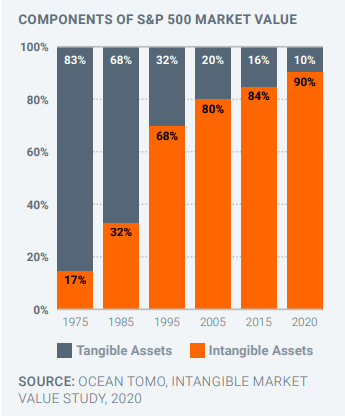
Take the United States. In 1975, 83 percent of the market value of its 500 biggest companies comprised “tangible” products and services, in areas such as manufacturing, agriculture and commodities. Much like Indonesia today. Today, the reverse is true. By 2020, 90 percent of the value of these companies came from “intangible assets”. Simply put, the most successful US companies make almost all their money through ideas, concepts, brands and innovative products and processes.
Advanced Asian economies – Japan, South Korea, Singapore, and Taiwan – have taken this path, moving over recent decades from agriculture to manufacturing to knowledge-based industries.
The Indonesian government and Asian Development Bank (ADB) recognize that the knowledge economy is vital for Indonesia’s future growth. “Building up knowledge-oriented sectors and processes is crucial for continued high growth and strengthening Indonesia’s position in ASEAN,” says Bindu N. Lohani, ADB’s vice president for knowledge management and sustainable development.
The Indonesian government also recognizes the importance of science and technology to economic development. The government enacted a new Science and Technology Law in 2019 and has also formed a new National Research and Innovation Agency (BRIN). Indonesia is increasingly investing in research and development (R&D) and innovation inputs, but it is not necessarily translating them into innovation outputs like patents, scientific publications, high-tech products, and trademarks. Indonesia ranks 87 out of 132 economies in the 2021 Global Innovation Index, which compiles relative performance in many of these areas.
IP drives partnership, tech transfer
For Indonesia to become a truly knowledge-based economy and achieve its economic ambitions, international partnerships are key, particularly with the private sector.
Few countries have developed thriving knowledge-based industries purely from domestic resources. Scientific knowledge, technological know-how, and the required R&D capital are dispersed globally. Innovative companies have generally moved away from the vertically-integrated model, where products are researched, designed, and manufactured in-house towards a globally networked model. This means multinational companies collaborate with small companies, academia, and the public sector at all stages of the R&D cycle, often across borders.
Indonesia’s challenge is to become a meaningful participant in this new world of networked innovation. It must attract innovative multinational companies to its shores, bringing with them the capital, skills, and technological know-how that Indonesia may be missing. Only through the international partnership will local companies upgrade their technical and innovative capacities and develop into meaningful innovators in their own right.
The importance of IRPs for Indonesia
For smaller Indonesian companies to be attractive partners for international investment and R&D partnerships, foreign investors need certainty over their intellectual property rights, especially clearly defined and easily enforceable patent rights. Evidence shows that robust intellectual property protection drives Foreign Direct Investment, with the OECD finding that a one percent increase in the strength of patent protection equates to a nearly three percent increase in FDI across all countries.
Invaluable sectors such as life sciences, and robust intellectual property rights are essential for local companies looking to partner with multinationals across the innovation value chain, from lab research, and clinical trials management to high-tech biopharmaceutical manufacturing. By allaying concerns about confidentiality, IP rights enable companies to share valuable proprietary information and know-how with local partners without worrying they are going to sacrifice their wider business objectives or lose control of their valuable assets. In turn, IP rights encourage the safe and efficient transfer of technology to local partners and the gradual development of skills and technical capacity.
In its Omnibus Job Creation Bill of 2020, the government recognized the importance of IPRs to foreign investment and job creation by amending laws relating to several different IP rights including patents. Meanwhile, the Directorate General of Intellectual Property continues to improve IP enforcement, addressing a particular weakness of Indonesia relative to its neighbors.
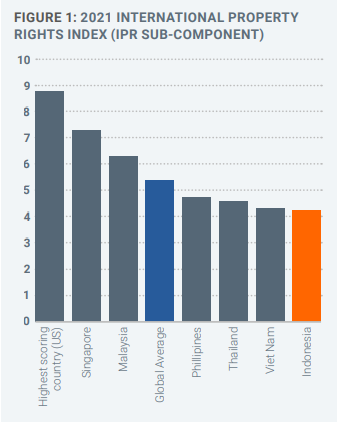
Nevertheless, relative to other SE Asian countries, Indonesia performs poorly across the spectrum of different IP rights (Figures 1 and 2). With some simple reforms, however, intellectual property rights can underpin Indonesia’s transition to a more knowledge-based, wealthy economy.
The importance of patents
As Indonesia looks to shift from low-value manufacturing towards value-added manufacturing and R&D activities in knowledge-based sectors, a strong framework for the protection and enforcement of patents is crucial. Patents are central to the business models of the highest-value industrial sectors, including life sciences, semiconductors, manufacturing of all kinds of electronic equipment and appliances, and natural gas extraction. In the European Union, for instance, patent-intensive sectors are responsible for 17% of all employment and 15% of total GDP.
A strong framework for the protection and enforcement of patents provides certainty to investors as they collaborate or license local companies, accelerating the process of technology transfer and capacity building. International comparisons, unfortunately, show that the quality of Indonesia’s patent system lags regional neighbors (Figure 3).
From an international perspective, the 2016 Patent Act, now under active reform, has been a particular area of concern. The Act introduced high levels of uncertainty and restrictions around patent rights in Indonesia. The Act continues to raise concerns about the ambiguity with which the government can override patents with a compulsory license, new rules that forbid patents for new uses of existing medicines, and requirements for medicines to be manufactured locally or forego patent protection. Other high-tech industries worry about new “localization” rules that would force them to transfer proprietary technologies to local companies.
A reform agenda for a regionally competitive patent system
The Omnibus Job Creation bill addressed some areas of concern raised by the 2016 Patent Act, notably Article 20’s requirement for a patented medicine to be manufactured locally within three years or risk compulsory license or cancellation of the patent. Now, importation or local licensing of the product is sufficient. Yet Indonesia still has some road to travel if its patent system is to be competitively regionally and beyond. Discussions continue within the government on how to further improve the patent environment. Here are three suggestions for reform.
1. Ensuring patents have meaningful terms
Patents have a 20-year life from the moment of filing. The clock starts ticking from that moment, meaning that the process of patent examination and medicines regulatory review eats into that patent term. Indonesia has taken steps to ensure patent examination timelines are not excessive, including through Patent Office reforms and the Patent Prosecution Highway with the Japan Patent Office. Nevertheless, examination delays of up to four years in Indonesia still persist.
Life science patents are further disadvantaged by the time taken for mandatory regulatory review by the National Agency of Drug and Food Control (NA-DFC). Thanks to delays here the median time to receive marketing authorization for new medicines in Indonesia is approximately 1600 days (4.4 years), among the longest in Asia. Collectively, such delays take a significant chunk of the patent term, undermining its value.
These delays hurt consumers and patients by delaying the market entry of new products, technologies, and medical treatments. OECD members Chile, Colombia, Costa Rica, Mexico, Korea, and the United States all have mechanisms in their patent laws to restore a portion of the patent lost due to unreasonable examination delays, commonly known as Patent Term Adjustment (PTA). PTA schemes ensure patent life is meaningful, providing certainty and stability for innovators, ultimately helping the patent system fulfill its social and economic role.
Similarly, many countries deploy a mechanism focused solely on pharmaceuticals, to restore some of the patent life lost due to mandatory regulatory review. So-called Patent Term Extension (Supplementary Protection Certificates in the EU) provides up to five year restoration for biopharmaceutical patent life during the process of regulatory review.
2. Eliminate improper patentability restrictions
Countries that are successful in innovation allow patents for all forms of the invention that meet patentability criteria, without discrimination by sector or technology. It is particularly important to eliminate improper patentability restrictions in the biopharmaceutical sector given the importance of follow-on innovation.
Follow-on innovations include refinements of existing technologies that can improve the efficacy and safety of existing medicines; new formulations and dosages that improve patient adherence and outcomes; and new uses for existing medicines. This is a particularly important form of innovation and is responsible for many of today’s important treatments (Figure 4).
Providing patent protection to follow-on innovation is important for the growth of pharmaceutical companies in emerging markets like Indonesia. Follow-on innovation can act as an entry into fully-fledged de novo drug R&D: young Indonesian companies could undertake proof of concept studies on existing molecules and license them out to more established R&D companies, or alternatively in-license molecules from established pharma companies, screen and validate them, and license them back to the parent companies for development. The management of clinical trials is also a growth area in Indonesia. Such business models can help the industry move up the value chain and in turn, generate high-quality jobs and sustainable economic growth. But they depend on appropriate patent protection.
Indonesia precludes patents on valuable biopharmaceutical inventions by adding an additional criterion for patentability that is inconsistent with international norms. Specifically, Indonesia requires a showing of “increased meaningful benefit” for certain biopharmaceutical innovations, which is inconsistent with the novelty, non-obviousness, and utility requirements of the WTO Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS Agreement).
3. Ensure patents can promote collaboration and technology transfer
The Covid-19 pandemic has illustrated the importance of patents for both innovation and the subsequent manufacturing of vaccines and therapeutics. By providing legal security over important commercial assets, patents played an important role in promoting Research and Development (R&D) collaboration between different organizations for Covid, often across borders and between competitors.
Once innovative Covid vaccines had been proven safe and efficacious IP rights, including patent rights, underpinned dozens of manufacturing collaborations allowing valuable proprietary manufacturing technology to be transferred to partners in multiple countries in a safe and sustainable way. As a result, there is now a global surplus of Covid vaccines.
Patent rights have also played a vital role in scaling up the manufacturing of innovative drugs for other public health challenges, notably HIV and Hepatitis C. Voluntary licensing of patents for these medicines to manufacturers in middle-income countries has underpinned a vibrant global market in high-quality low-cost generic medicines while providing significant local industrial benefits via the local partnering companies.
Indonesia’s 2016 Patent Law however discourages these voluntary forms of IP sharing, mainly through its facilitation of compulsory licensing on grounds that are opaque or inconsistent with its international obligations under the TRIPS Agreement. The issuing of a compulsory license for Covid therapeutic remdesivir, despite the existence of a voluntary license, is one example of the international investment risk of Indonesia’s patent environment.