By Mark Schultz & Jaci McDole
Summary
- The percentage of patented medicines on the EML has steadily risen in recent years but remains relatively small. As of January 2020, 47 out of the 458 total items (10.3%) listed on the 21st edition EML are under patent somewhere in the world.
- 20% of lower income countries surveyed do not have any active patents listed for the 47 patented medicines on the 21st 80% of lower income countries have 50 or fewer active EML drug patents.
- Approximately 20% of all active EML patents in lower income countries are subject to an MPP license, bilateral license, and/or commitment to not enforce, with commitment to not enforce accounting 25% of that group.
- Cancer treatments have remained within the top three priorities for new additions to the EML throughout the three most recent editions of the EML.
- The majority of medicines submitted by known sources for the 20th (2017) and 19th (2015) EMLs came from NGOs or the WHO or its affiliates.
1. Introduction
Every two years the World Health Organization (WHO) publishes its Model List of Essential Medicines (EML), which identifies drugs and biologics “deemed essential for addressing the most important public health needs globally.” Innovation in the life sciences is constant and public health needs evolve, so new medicines are added to each edition of the EML while others are removed.
The EML occupies an important role in public health policy. First, it is a source of guidance and a measuring stick for public health systems as to the drugs and other treatments they should have available for patients. Second, it is a focus for public health policy discussions about which treatments should be prioritized and how well public health systems are meeting the goal to make them available.
Since the EML plays this important role, the availability and accessibility of drugs on the EML is a frequent topic of policy discussion. This policy discussion often addresses the patent status of drugs on the EML. Of course, nearly all of the drugs on the EML started out as patented; all of them eventually come off patent, either before or after being listed on the EML. Patent status is sometimes viewed as a proxy for affordability, but in reality, things are not that simple, as many patented drugs on the EML are subject to institutionalized access programs to ensure availability and affordability in developing countries.
Trends regarding the EML are thus of great interest and relevance to public health policy discussions. This paper measures and addresses three trends in recent editions of the EMLs:
- The percentage of drugs and biologics on the most recent publication of the EML (21st edition, 2019) currently under patent in lower income countries.
- The availability of institutionalized access programs, such as the Medicines Patent Pool and other initiatives to make medicines broadly available in low-income countries.
- Trends regarding which types of health issues (e.g. cancer) are addressed by additions to the EML and who is proposing such changes, over the last three editions of the EML.
Our methodology builds upon the one employed by Reed Beall and Amir Attaran’s 2016 study for the World Intellectual Property Organization, Patent-based Analysis of the World Health Organization’s 2013 Model List of Essential Medicines. Our methodology differs, however, in that we were able to make use of several public databases that have become available since Beall and Attaran did their study. We explain our methodology in detail in Appendix A.
In the interest of making a timely contribution, we forego Beal & Attaran’s step of verifying patent status through contacting pharmaceutical companies. However, we were able to use the MedsPal PatInformed database, which was developed by WIPO with the input of a large portion of the innovative pharmaceutical industry to perform a similar check.
2. Recent Trends in Patent Status for EML Medicines
Every other year, the WHO revises its Model List of Essential Medicines (EML) adding some medicines and removing others as standards of care advance. In recent editions of the EML, the length of the list has grown significantly, from 373 in 2013 to 458 in 2019 as additions have outpaced removals. Some (although not the majority) of each year’s new additions to the EML have still been under patent.
This Section provides a look at the basic trends regarding patent status on the EML, then takes a more in-depth look at where those patents are filed in lower income countries, and finishes with an overview of some available information regarding institutionalized access programs for these medicines, including low cost licenses and commitments not to enforce.
2.1 Basic Trends Regarding Patented Drugs on the EML
The percentage of the EML composed of drugs under patent has increased in recent years. They remain a relatively small plurality of the drugs on the list, but they now account for 10.3% of the 21st Edition of the EML as of May 2020, as opposed to 5.4%, upon publication of the 18th Edition of the EML in 2013.
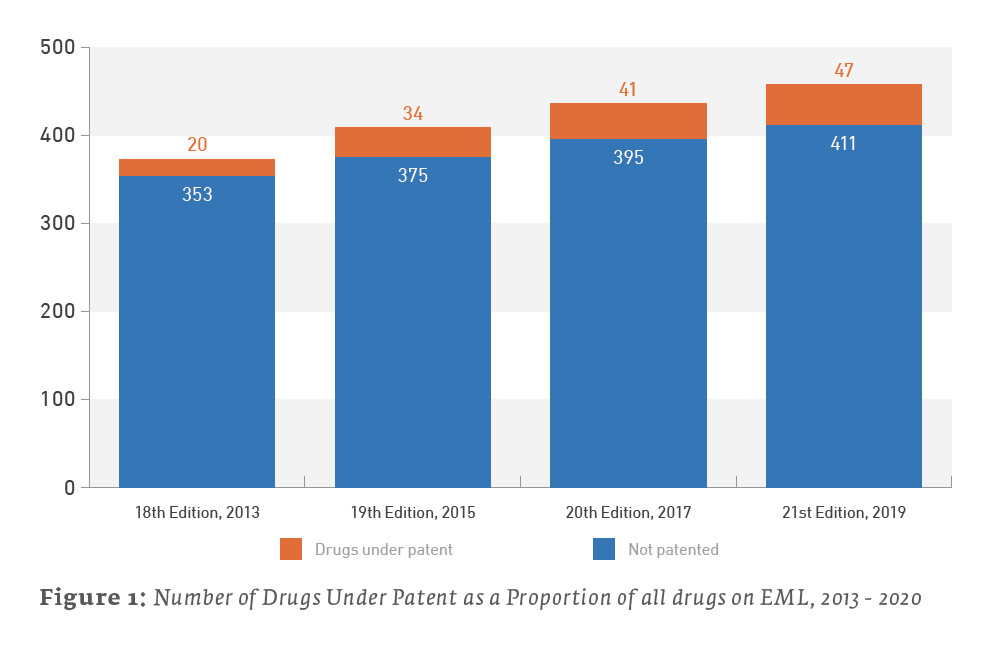
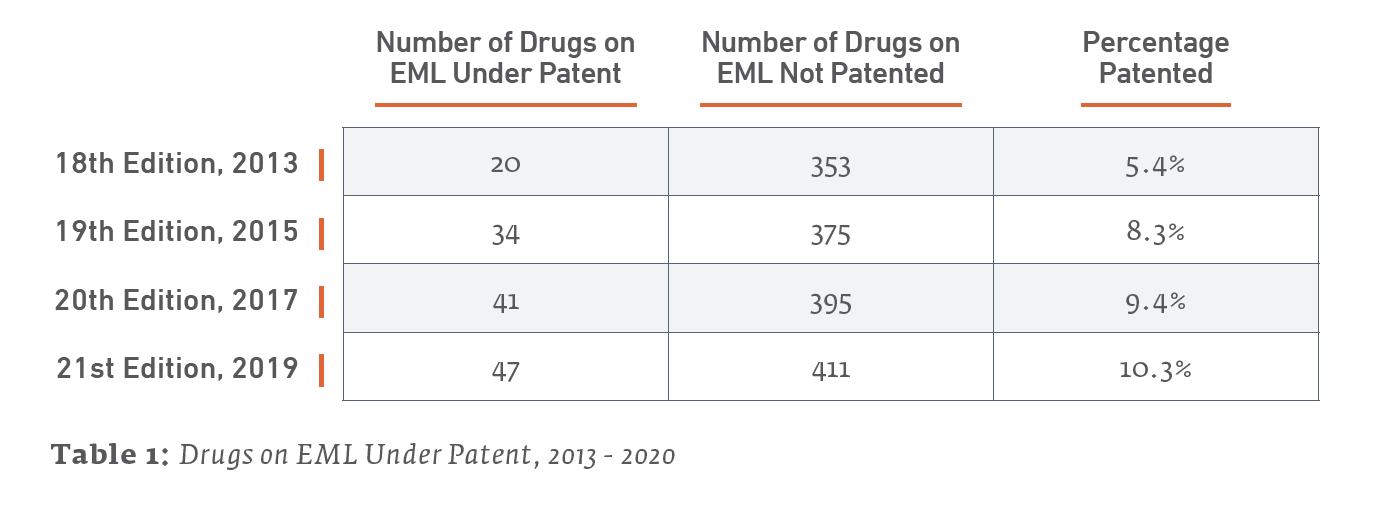
The EML and Patents by the Numbers
Only 47 out of the 458 total items (10.3%) listed on the 21st edition EML may be considered under patent as of January 2020. A review of the 20th edition EML found that 41 out of 436 items (9.4%) listed on the 2017 EML were under patent somewhere in the world. Therefore, the 2019 EML is up a net 6 patented drugs from the 2017 EML. (We say “net” due to patent expirations and removals from the list.)
The following Table 2 further details recent trends in patent status of medicines on the last 4 editions of the EML, going back to 2013. As previous studies have done (e.g., Beall & Attaran 2013), a drug counts as under patent if it is patented somewhere in the world, with a focus on lower income countries.
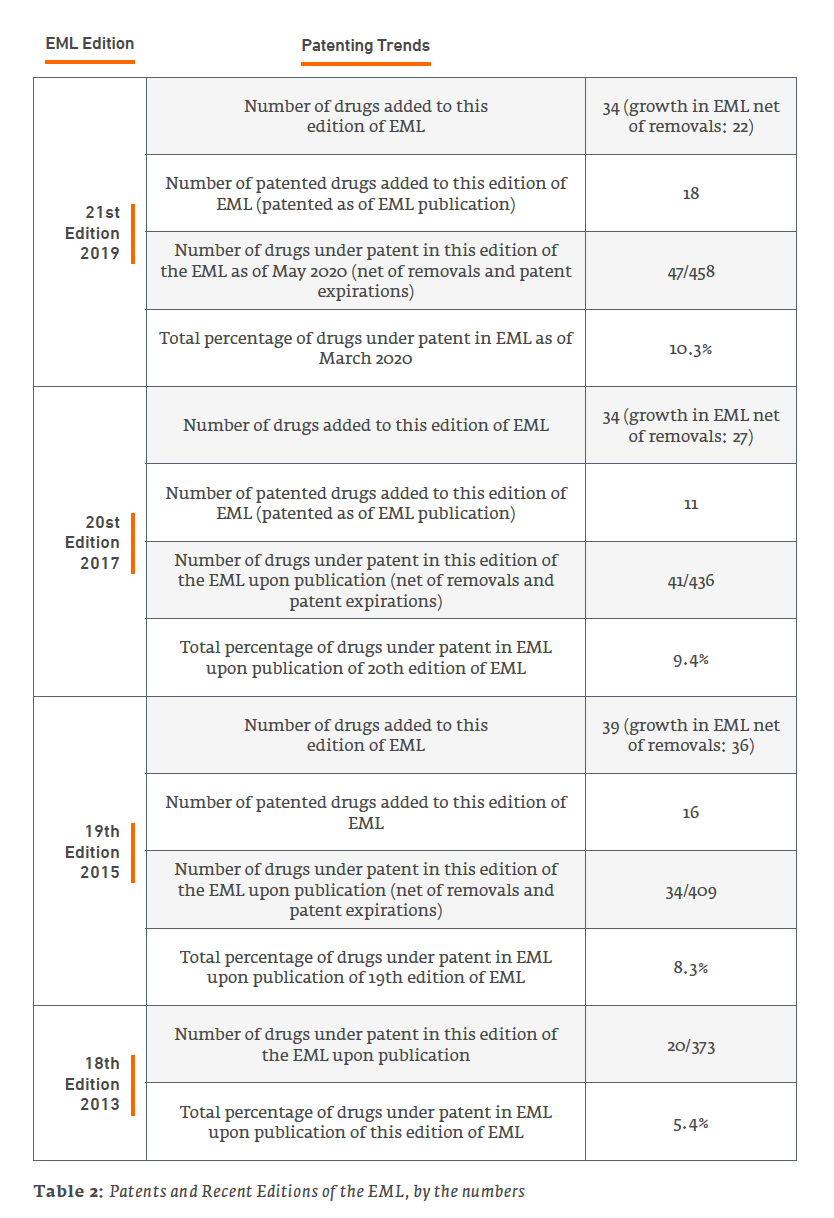
Patents and the EML: A Dynamic Relationship
The reader should keep in mind that patents have a limited term, so the number of patented drugs on the EML is not simply cumulative – although new, patented drugs are added with each edition, at least some earlier patents expire between editions of the EML. But for the addition of new, more-recently patented drugs in new editions of the EML, the percentage of drugs under patent on the EML would decline over time until reaching zero.
An example of the ever-changing makeup of patented drugs on the EML can be illustrated by comparing the patent status on drugs in the 2019 21st Edition of the EML to the 2013 18th Edition.
First, the following drugs that were on the 18th Edition and, at the time of that edition, counted as patented, remain on the 21st Edition EML, but the last patents appear to have expired, and they no longer contribute toward the patent percentage (note that this is just a snapshot comparing these two editions; there have been other interim changes in status as well):
- Artemether + lumefantrine
- Bevacizumab
- Lamivudine + Nevirapine + Zidovudine
- Oseltamivir
- Pegylated interferon alfa 2a
It is notable that two of these drugs, bevacizumab and pegylated interferon alfa 2a, appear to have gone off patent as recently as 2019. They still counted as under patent at the time of publication of the 21st Edition of the EML, but no longer count as of the publication of this paper. Patent status is always changing.
Second, another way to illustrate the ever-changing nature of patent status on the EML is to consider the cumulative number of patented drugs added to the list versus the current number of patented drugs still on the 21st Edition of the EML. Since the 18th Edition of the EML studied by Beall and Attaran (which serves as our baseline), included 20 patented drugs, 45 patented drugs have been added to the list. However, the current number of drugs on the list is 48 due both to patents expiring and to some drugs being removed from the list.
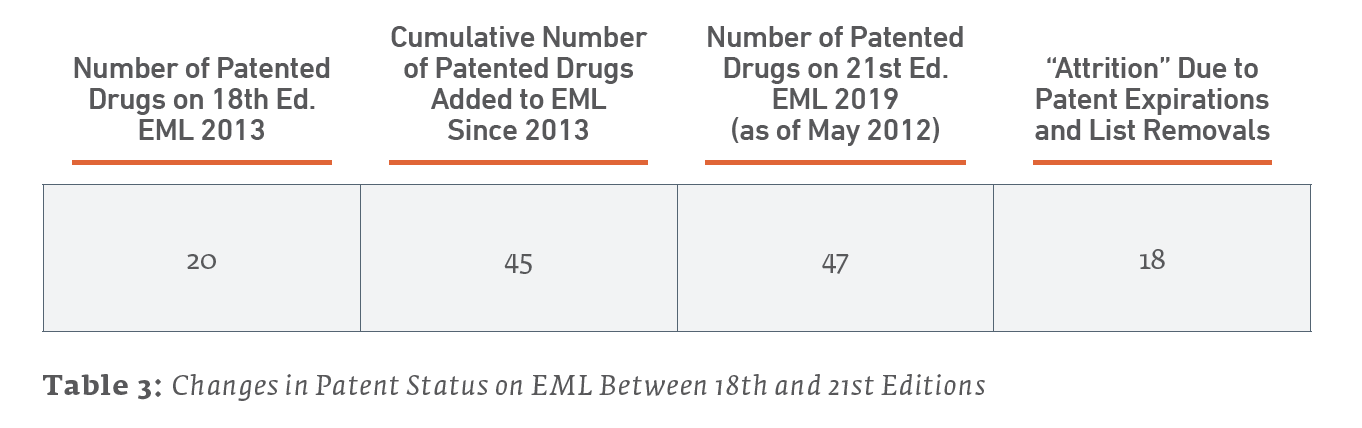
What these examples illustrate is the dynamic nature of the EML and patent status. The vast majority of drugs on the EML were under patent for a time when first marketed. Those patents may have expired before or after placement on the EML (or have yet to expire). Any item on the EML is subject to replacement as the standard of care advances, and a replacement may or may not still be under patent for a time.
Inevitably and eventually, today’s patented drugs on the EML will no longer be tomorrow’s, either due to patent expiration or replacement with another drug as standards of care advance.
What Kinds of New Treatments on the EML are Patented?
The evolution of standards of care is why patented drugs are placed on the EML. Each new drug represents an opportunity to provide a better treatment or one where none existed before, or a chance to address a new or growing public health problem. As scientific research advances, new treatments are added to the existing, wide-ranging arsenal used to combat constant health concerns such as cancer, MDRs, NCDs, and HIV/AIDS. The newest ones are still under patent.
Examined in this light, it is useful to consider what the newly-added patented drugs are used to treat. Of the 18 patented drugs added to the 21st Edition of the EML in 2019:
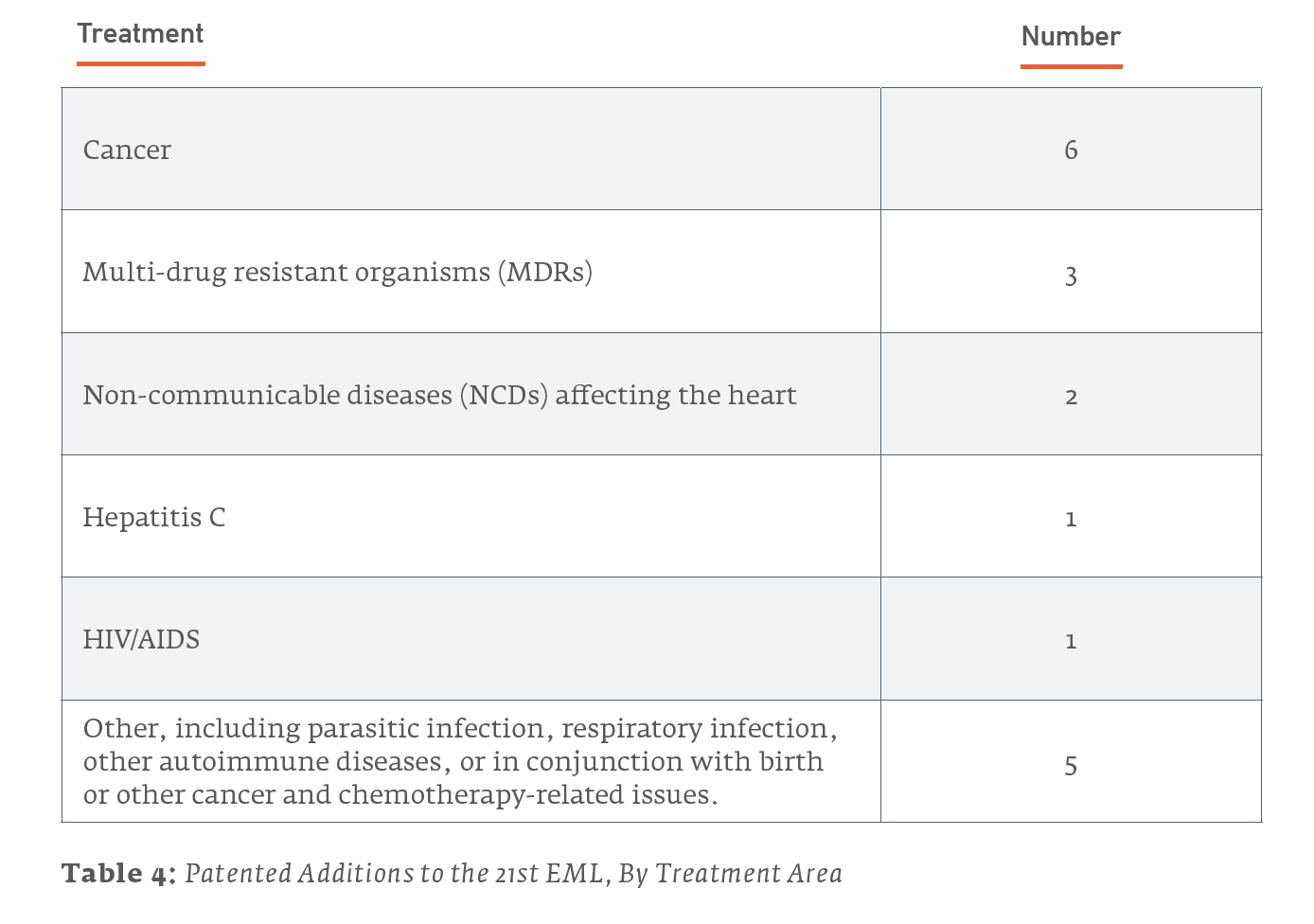
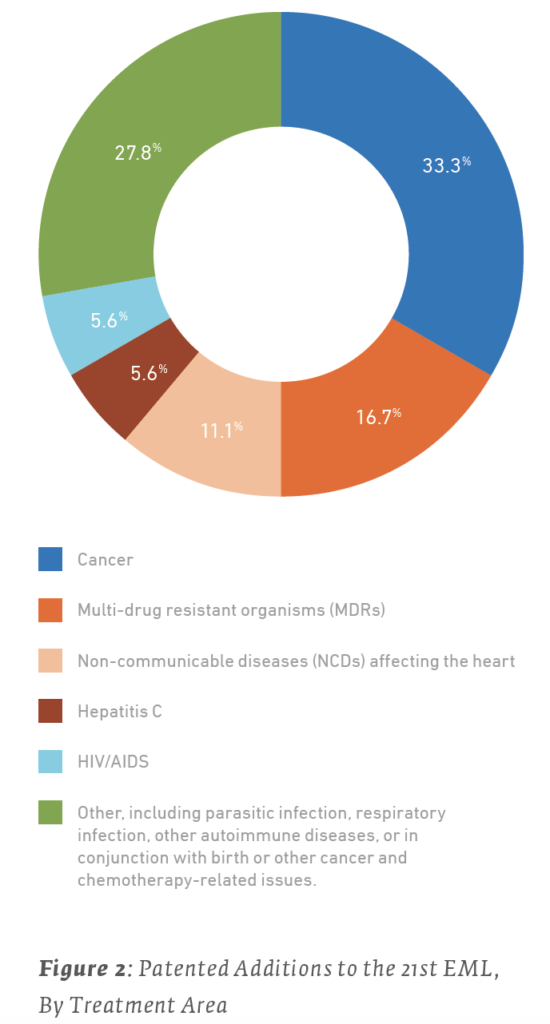
However, for most of these health issues, non-patented treatments were also added to the list. The majority of treatments added to the 21st EML were not patented, as illustrated in Table 5 and Figure 3.
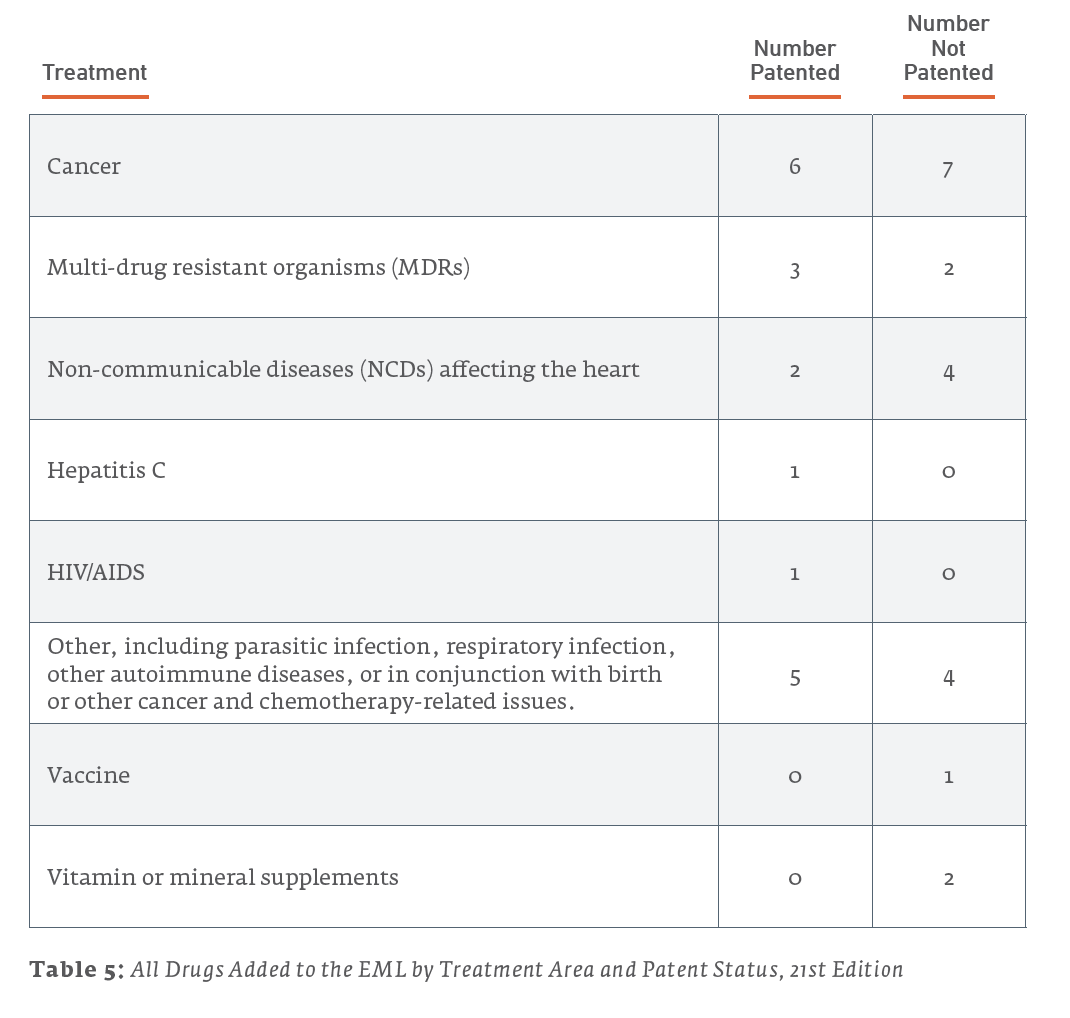
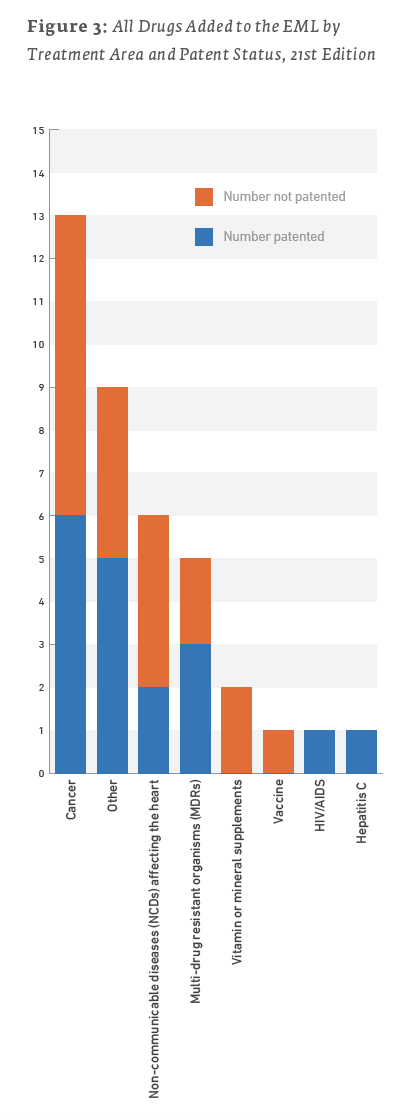
It is difficult to draw overarching conclusions from these statistics. However, a few points are notable:
- As life expectancies improve globally, cancer and non-communicable diseases, such as heart disease, have become a greater focus of global health policy. With new priorities come new challenges, and sometimes newer drugs, some of which are likely to be patented.
- Some breakthrough treatments, such as cures for Hepatitis C, tend to be embraced sooner rather than later, and thus while still under patent.
- Emerging and urgent problems, such as treatments for multi-drug resistant organisms, are urgent problems demanding new (and thus more likely to be patented) treatment.
The basic trends illustrated here only tell part of the story. Just because a medicine is subject to a patent somewhere in the world does not necessarily mean that it is patented everywhere. For some of these drugs, there are relatively few patent filings worldwide, especially in lower income countries. Moreover, there are a number of institutionalized solutions to promote greater access to these medicines. We discuss these trends in the next two sections.
2.2 EML Patent Status in Lower Income Countries
While the takeaway for policy discussions from previous studies of the patent status of medicines on the EML (Attaran 2004; Cavicchi & Kowalski 2011; Beall & Attaran 2016) has been the percentage of medicines on the EML under patent somewhere in the world, the picture is much more complex than that simple number. (As those previous studies discuss).
Just because a medicine is under patent somewhere, does not mean it is under patent everywhere. Of course, patents in some countries matter more than others – when a country does not have capacity to produce a medicine, then patent status in potential supplier countries may be of greater consequence.
We therefore consider in greater detail which drugs are patented in which countries and when those patents expire. We focused on 150 lower income countries and obtained most of our data from the Medspal online database supplemented by data from DrugPatentWatch as further explained in our methodology discussion in Appendix A.
Here are some statistics that give a view of the nature of less-than complete patent coverage of EML drugs that are under patent (somewhere). Of the 150 lower income countries we examined:
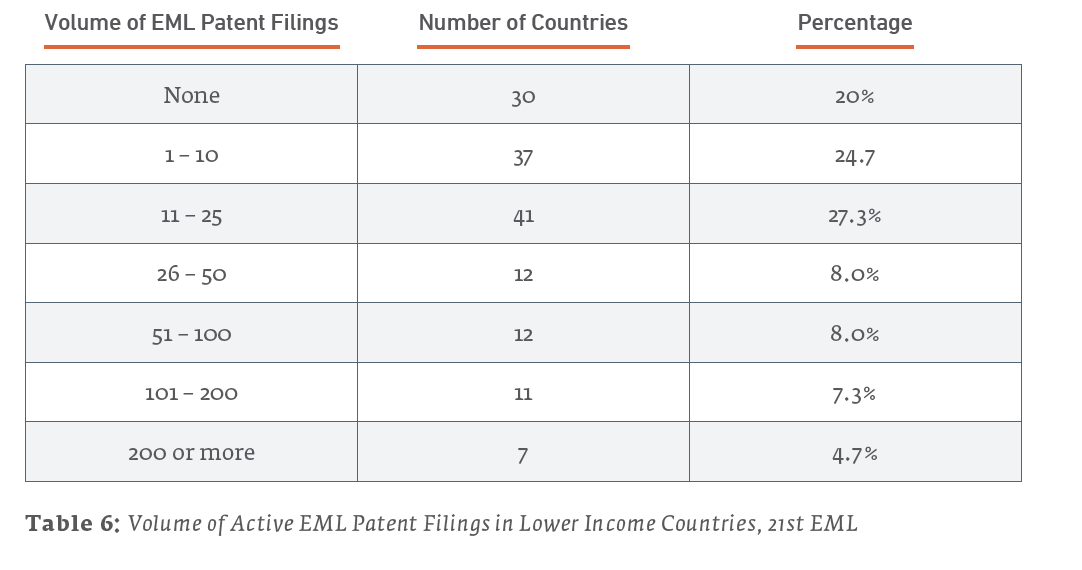
A few key facts about EML patent filings in lower income countries:
- There are approximately 6,500 total active filings within the reviewed jurisdictions for the medicines surveyed
- A significant majority of lower income countries have 50 or fewer total active patent filings. Overall, 120 (80%) of the 150 lower income countries reviewed during this study have 50 or fewer total active patent filings.
- Approximately 2% of the EML patent filings in lower income countries are set to expire in 2020.
- The seven countries with 200 or more active patent listings in order from greatest to least are China, the Russian Federation, Mexico, the Democratic Republic of Korea, Brazil, South Africa, and Turkey with 657, 418, 400, 356, 293, 239 and 223 total active patents, respectively.
Before drawing conclusions about these statistics, one arguably should also consider MPP and bilateral licenses or commitments not to enforce, which we discuss later.
Table 7 provides a more detailed summary, listed by drug. Further details, with country-by-country breakdowns are provided in Appendix B, which is available online on the Geneva Network website as well as in the version of this paper posted on scholarly databases.
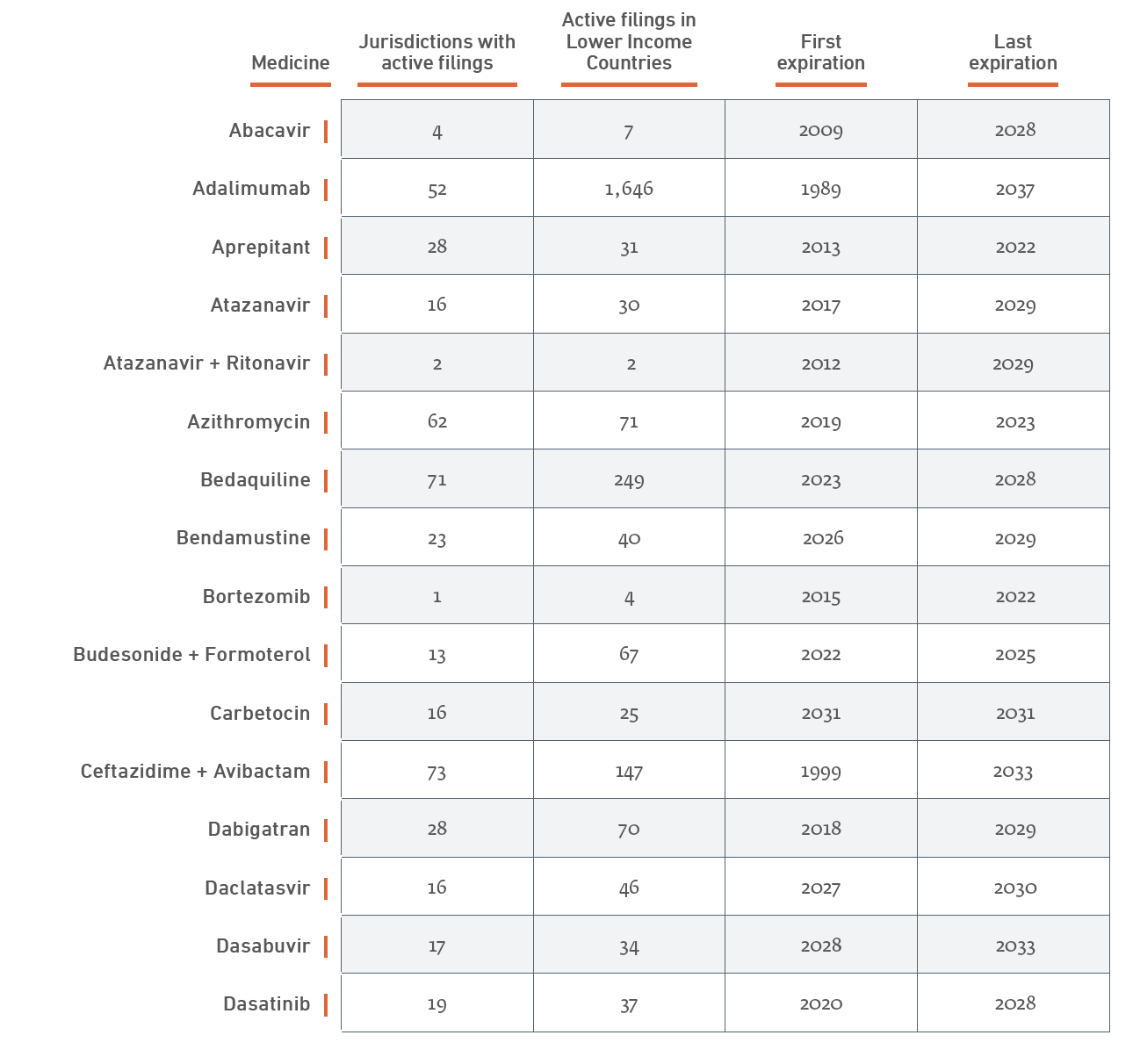
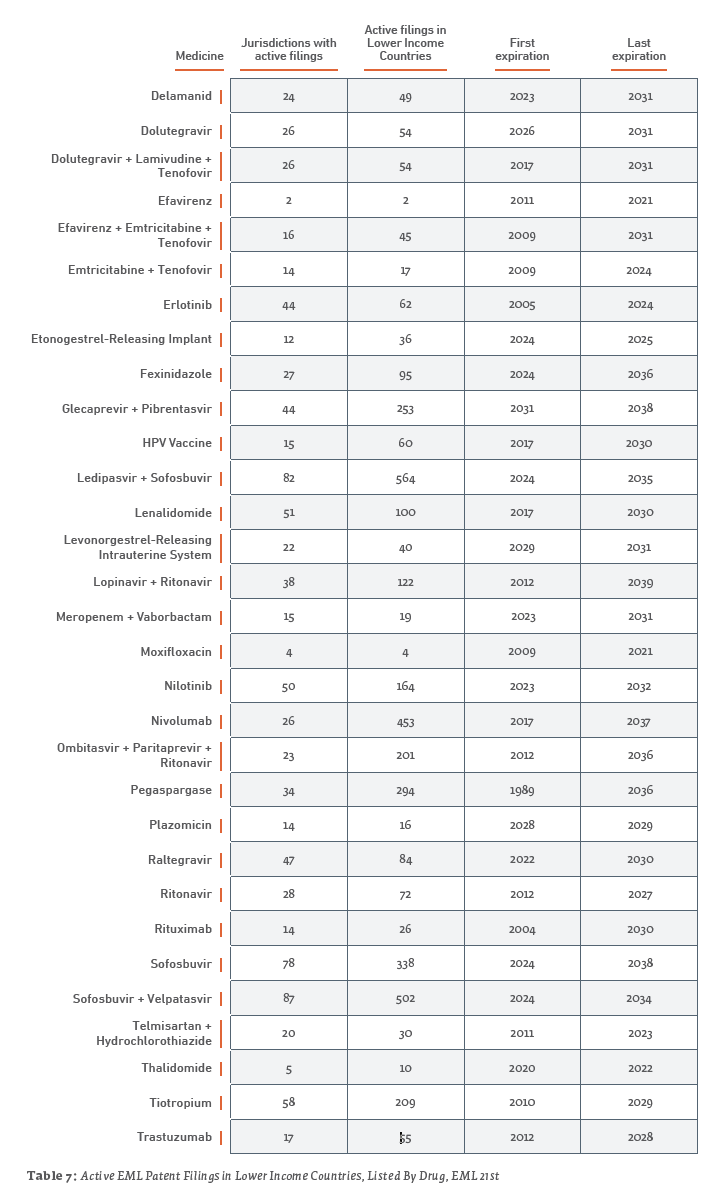
2.3 Institutionalized Access Programs and Commitments
When considering the role that patents might play with respect to access to drugs, one concern voiced by some is that the existence of a patent gives the owner pricing power. However, in the case of patented drugs on the EML, patent owners often make commitments to limit their pricing power voluntarily. Medicines Patent Pool (MPP) and bilateral licenses are two ways manufacturers and NGOs are working to ensure access to already developed essential medicines in lower income and underserved countries. Likewise, endeavors such as Medicines for Malaria Venture (MMV) oversee the research and development of affordable, essential medicines. Some manufacturers even choose to sign a commitment to not enforce their IPRs for certain medicines in specific regions. These are just some of the methods used to increase access to essential medicines.
The extent of such commitments is not always well documented, but thanks largely to the MedsPal online database we were able to determine that an MPP license, bilateral license, and/or commitment to not enforce is known to exist for 16 of the 37 medicines currently on the EML and subject to a patent:
- Atazanavir
- atazanavir+ritonavir
- bedaquiline
- daclatasvir
- delamanid
- dolutegravir
- dolutegravir+lamivudine+tenofovir
- efavirenz+emtricitabine+tenofovir
- emtricitabine+tenofovir
- glecaprevir+pibrentasvir
- ledipasvir+sofosbuvir
- lopinavor+ritonavir
- ritonavir
- sofosbuvir
- sofosbuvir+velpatasvir
- tenofovir
MPP and bilateral licenses allow for the manufacture of generics to better accommodate regional medicinal needs. Approximately 20% of the estimated 6,500 active filings in lower income countries are subject to an MPP license, bilateral license, and/or commitment to not enforce, and one-fourth of those are commitments to not enforce. In fact, all active patent filings in the surveyed jurisdictions for lopinavir+ritonavir and ritonavir are subject the commitments to not enforce.
3. Trends and Changes in Use and Submissions to the EML
We also examined trends regarding the types of treatments being placed on the EML, as well as where submissions to the EML originated from. This information is a new contribution to the research and policy discussion regarding the EML motivated by increasing interest in this topic expressed in the policy community. The trends we found show the dynamic nature of the EML as well.
3.1 Trends in Treatments Added to the EML, 2013 – 2019
While certain health concerns, such as cancer, MDRs, NCDs, and hepatitis, have consistently been addressed through new additions for the past three EML publications, no single use has consistently topped the list. However, cancer (as a general category) has remained within the top three uses addressed across the 19th, 20th, and 21st editions of the EML. For both the 21st and 19th editions, the majority of new additions were drugs used to treat cancers, while MDRs topped the list for the 20th edition EML.
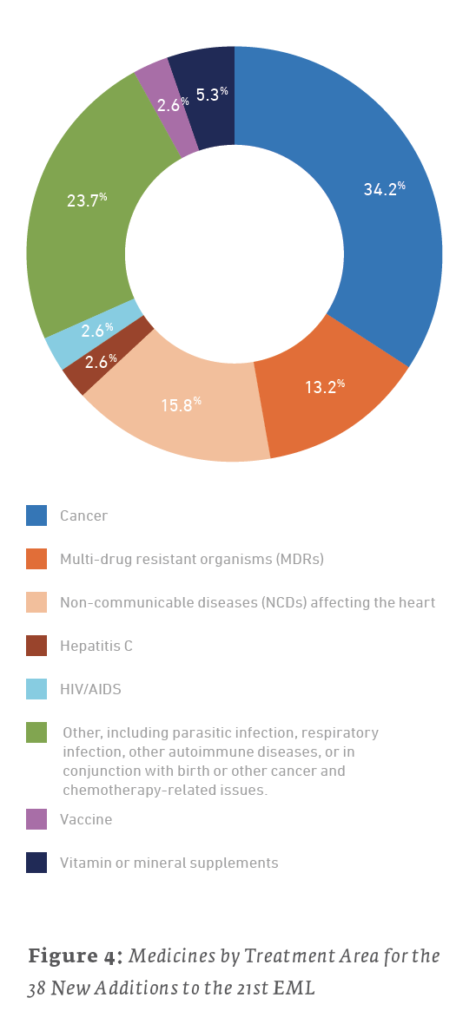
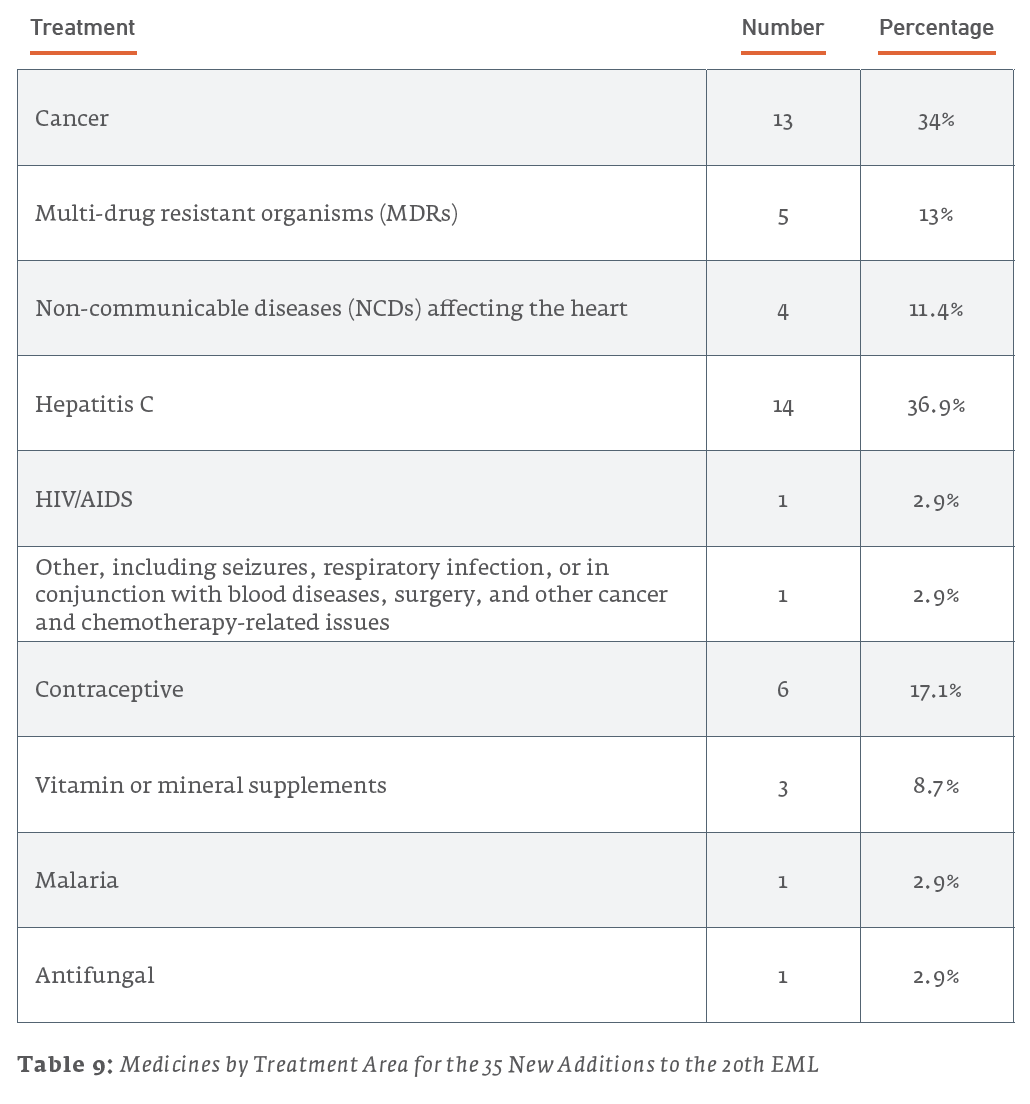
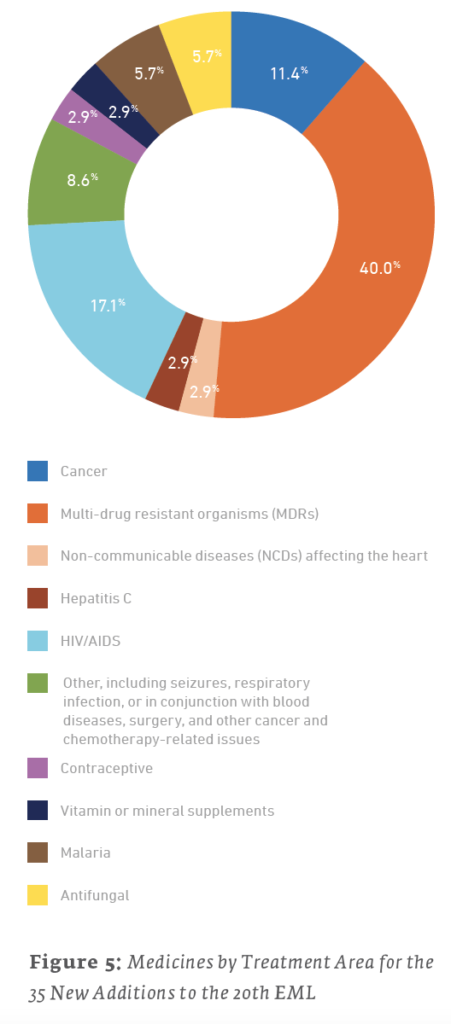
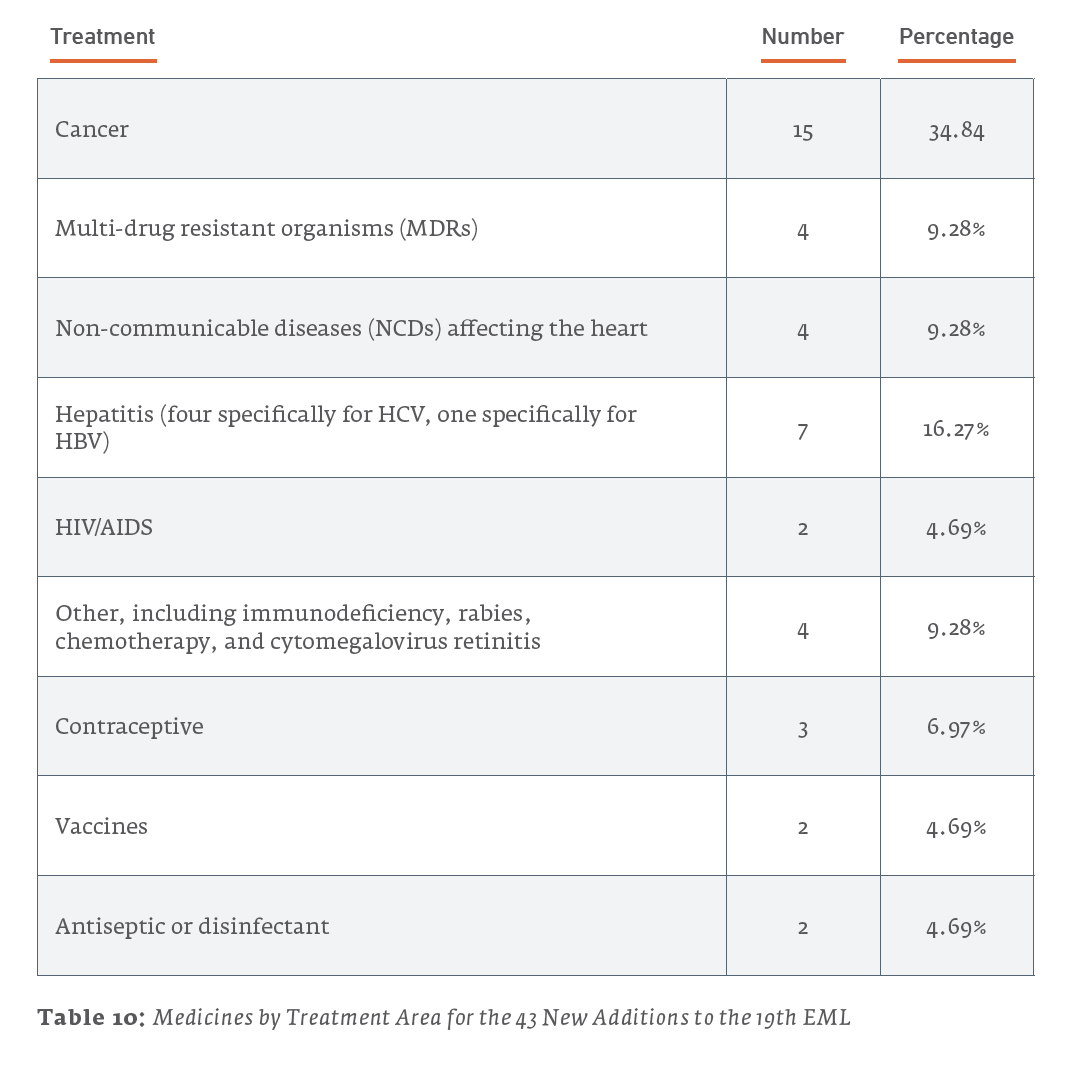
Of the 38 new additions to the 21st edition (2019) EML, 13 (34.2%) are used for treating cancers, 6 (15.8%) are for treating NCDs affecting the heart, and 5 (13.2%) are for treating MDRs. For the 35 new additions to the 20th edition (2017) EML, 14 (40%) are for treating MDRs, 6 (17.1%) are for the treatment of HIV/AIDS, and 4 (11.4%) are for treating cancers. The 43 new additions to the 19th edition (2015) of the EML saw 15 (34.9%) drugs for treating cancers, 7 (16.3%) drugs for the treatment of hepatitis, and a tie for third with 4 (9.3%) drugs each for drugs treating NCDs affecting the heart and MDRs.
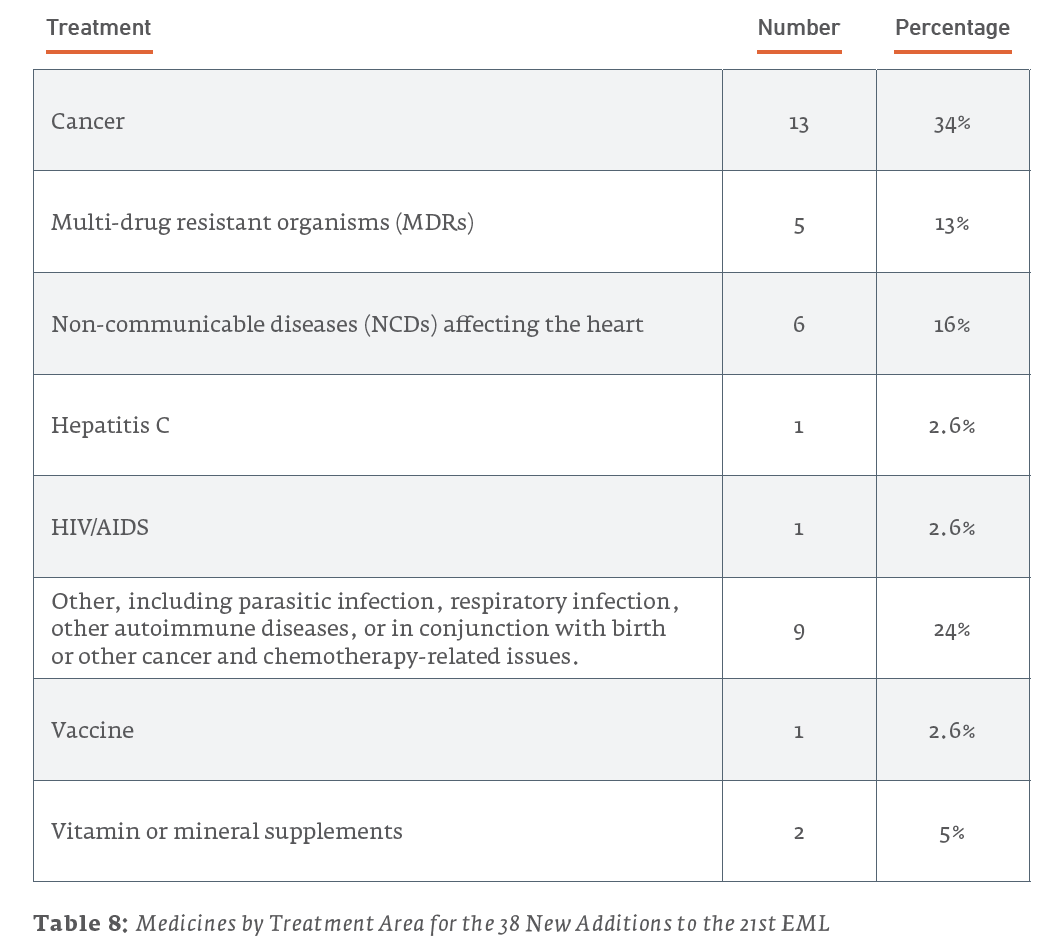
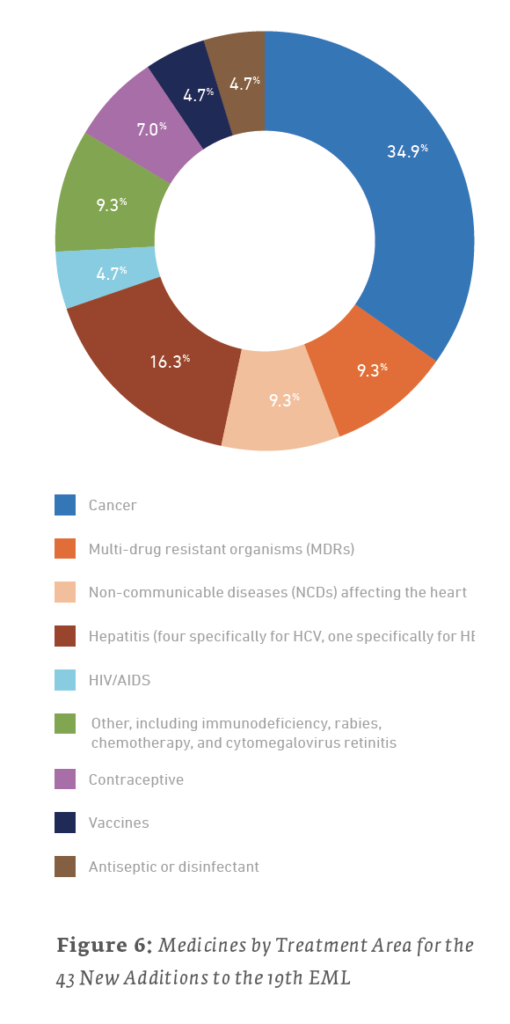
3.2 Who is Submitting New Drugs to the EML?
Although information relevant to each new addition’s use was easily attainable, determining who submitted the various medicines for consideration to add to the EML was rather difficult. Data provided on the WHO website pertaining to submissions is inconsistent, incomplete, and does not cover all new additions. Also, as of this writing, no information is yet readily available concerning any submissions for the 38 additions to the 21st EML (2019).
Here are a few key facts are apparent regarding who submitted drugs for consideration, and successfully listed, on the 2015 and 2017 editions of the EML:
- For both the 20th (2017) and 19th (2015) EMLs, either NGOs or the WHO and its affiliates made the majority of submissions, for cases where sources are known.
- In both editions, approximately 10% of all new additions were submitted by the drug’s manufacturer.
- In 2017, two submissions were developed in conjunction with MMV, an R&D organization dedicated to “developing and facilitating delivery of new, effective and affordable antimalarial drugs.”
- Two medicines, bedaquiline in 2015 and sofosbuvir + velpatasvir in 2017, were each separately submitted by both the manufacturer and the WHO.
Here are a few further details regarding submitters:
- Out of the 6 medicines listed for the treatment of HIV/AIDS, 5 were submitted by the WHO, and 1 was jointly submitted by the NGOs GAFFI and ILDS.
- Out of the 4 medicines listed for treating cancers, 3 were submitted by the NGO UICC, and 1 was submitted by an academic/practitioner.
- Out of the 2 medicines listed for treating malaria, both were submitted by their manufacturers and developed in conjunction with MMV.
- Both of the medicines listed for antifungal treatment were submitted by NGOs; 1 was submitted solely by GAFFI, and 1 was submitted jointly by GAFFI and ILDS.
- The 1 medicine listed for the treatment of hepatitis was separately submitted by both the WHO and the manufacturer.
- Out of the 3 medicines listed for other treatments, the 1 concerning seizures was submitted by the WHO, the 1 concerning respiratory infections was submitted by an academic/practitioner, and the last 1 was submitted via the “Other” category.
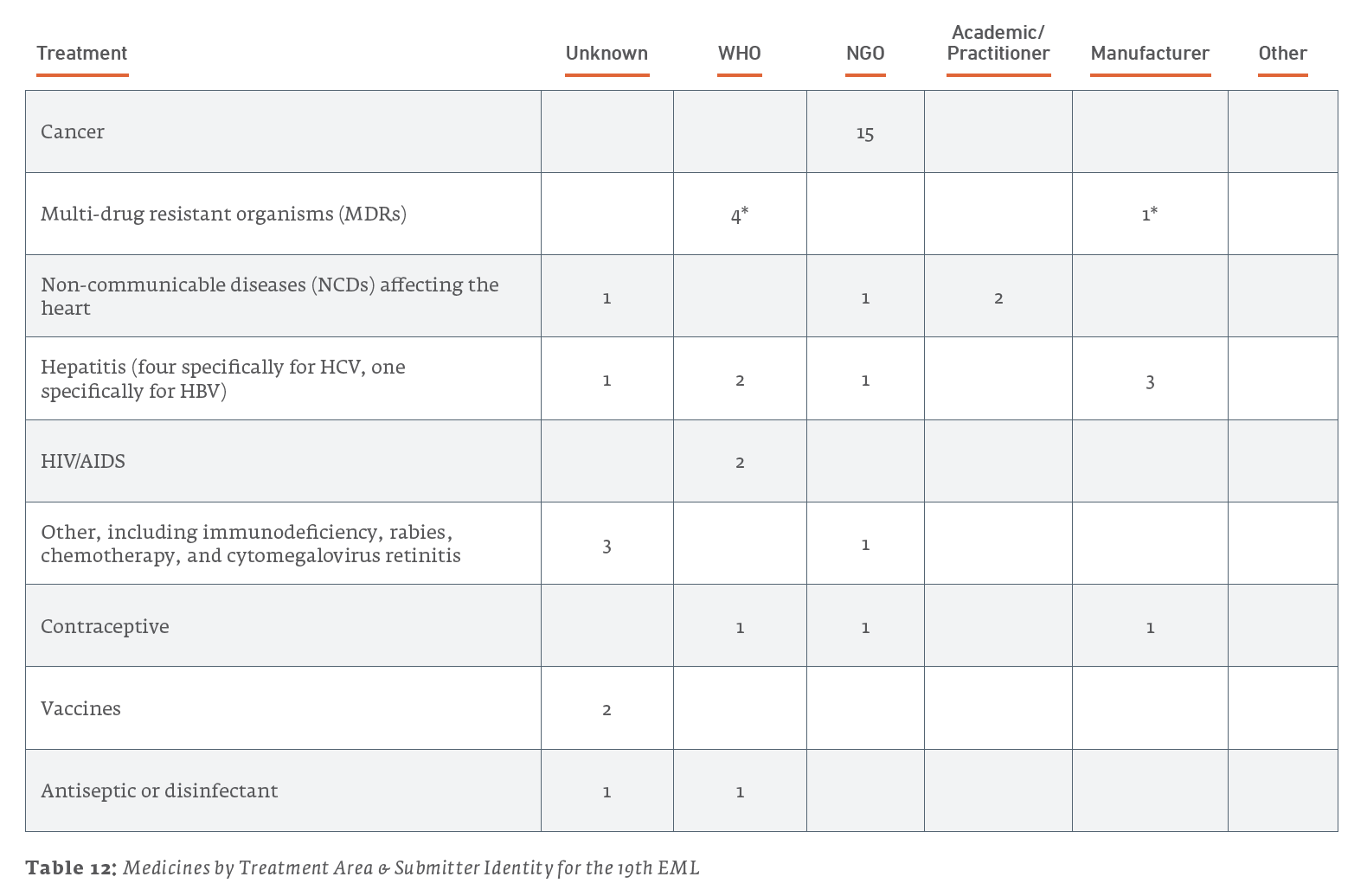

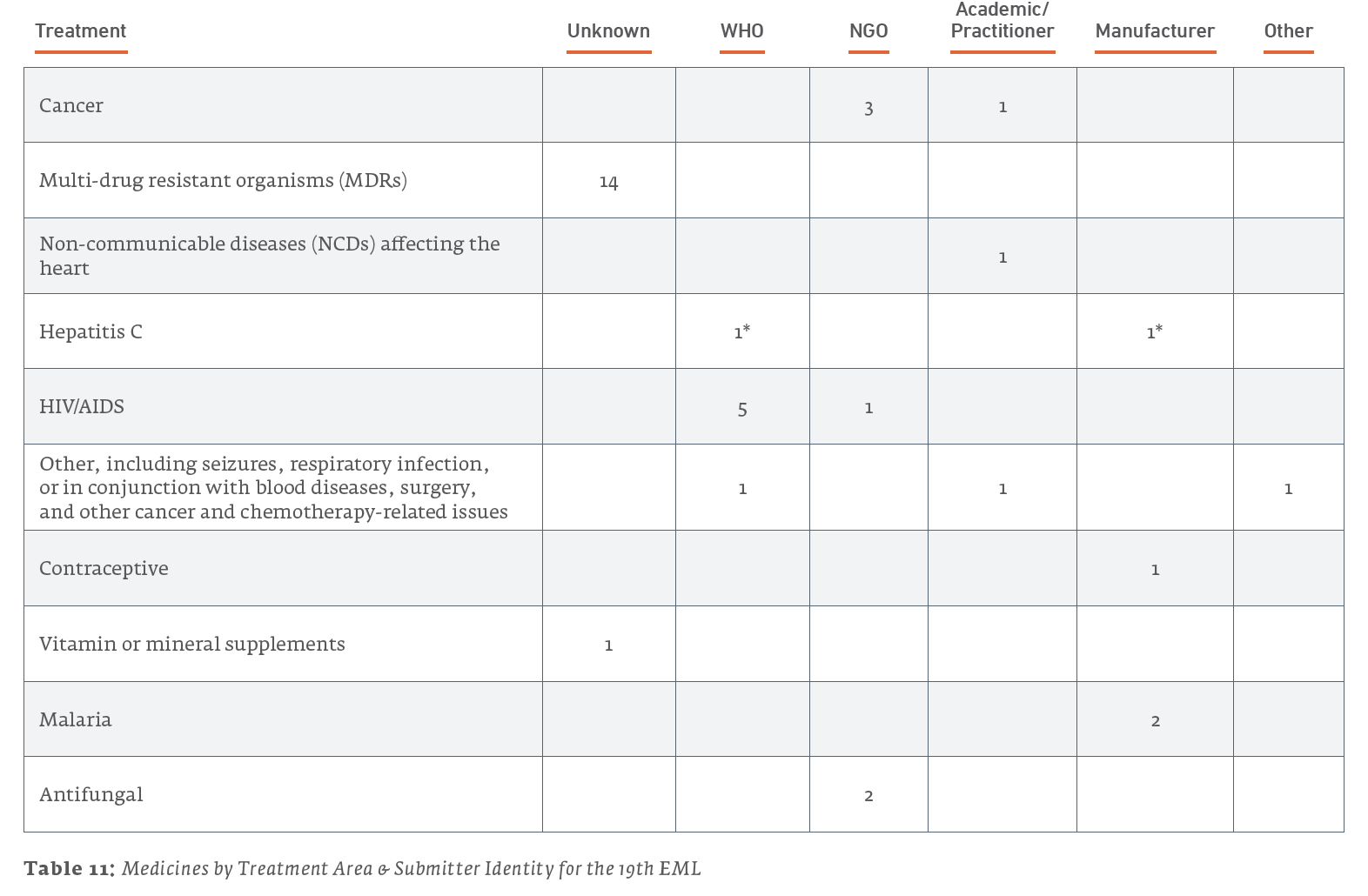
Here are a few further details regarding submitters:
- Out of the 15 medicines listed for treating cancers, all 15 were submitted by the NGO UICC.
- Out of the 7 medicines listed for the treatment of hepatitis, 3 were submitted by their manufacturers, 2 were submitted by the WHO, 1 was submitted by the NGO MSF, and 1 was of unknown submission.
- Out of the 4 medicines listed for other treatments, 3 were of unknown submissions, and the 1 concerning antiviral treatment of cytomegalovirus retinitis was submitted by the NGO MSF.
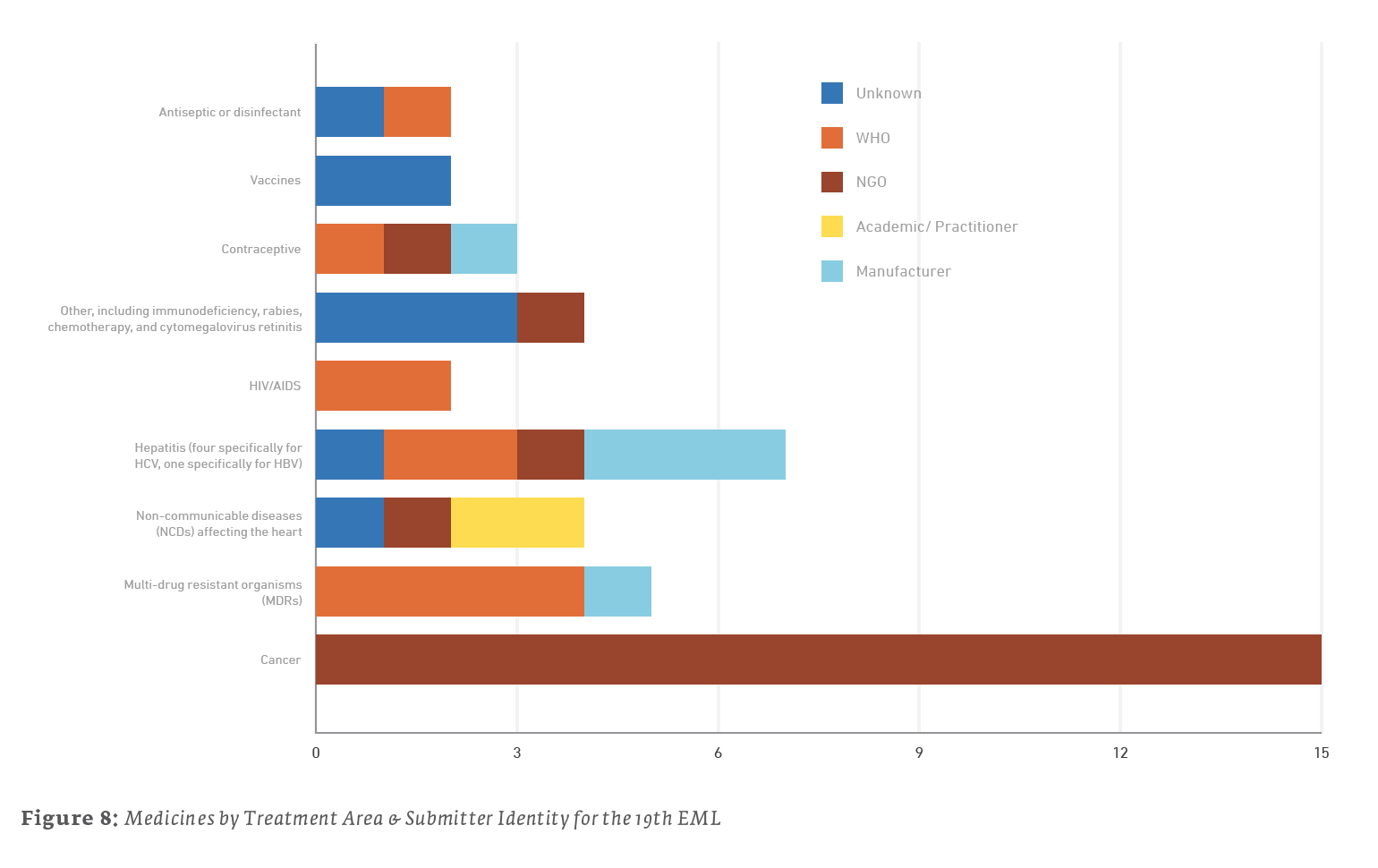
Trends in new additions reflect the EML’s adaptability are based on global health needs, but they also highlight persistent problems such as cancer and MDRs. Likewise, most of the drugs and biologics submitted for consideration within the EML appear to be submitted by those “on the front lines,” the WHO and those NGOs studying these problems and looking for solutions.
Conclusion
While the number of patented medicines on the EML has increased in recent editions, the portion of the list currently under patent remains a small portion of all drugs on the EML, currently about 10%. A deeper dive into the data shows that many drugs are only patented in a fraction of lower income countries. Thus, 80% of lower income countries have 50 or fewer active patent filings on that ten percent. Moreover, many of these patented drugs are subject to institutionalized programs to provide access at lower cost. This paper provides an update to previous efforts to understand the nature of the EML, while expanding previous information thanks in part to the existence of new freely accessible online databases showing patent status and participation in programs to provide access.
Bibliography
Attaran A, L Gillespie-White. (2001). Do patents for antiretroviral drugs constrain access to AIDS treatment in Africa? JAMA 286(15):1886-92.
Attaran A. (2004). How Do Patents And Economic Policies Affect Access To Essential Medicines In Developing Countries? Health Affairs, 23(3):155-66.
Beall, R. & Attaran, A. (2016). Global Challenges Report: Patent-based Analysis of the World Health Organization’s 2013 Model List of Essential Medicines.
Cavicchi J.R., & Kowalski, S. (2009). Report of Patent Literature, Search Methodology and Patent Status of Medicines on the WHO EML. International Technology Transfer Institute, Franklin Pierce Center for Intellectual Property.
Cavicchi J.R., & Kowalski, S. (2011). Preliminary Report on Search Methodology and Patent Status of Medicines Added to the WHO EML from the 18th meeting of the WHO Expert Committee on the Selection and Use of Essential Medicines. University of New Hampshire.
Medicines Patent Pool. (2020). https://www.medspal.org/
World Health Organization Model List of Essential Medicines, 21st List, 2019.
Geneva: World Health Organization; 2019. https://apps.who.int/iris/bitstream/handle/10665/325771/WHO-MVP-EMP-IAU-2019.06-eng.pdf?ua=1
World Health Organization Model List of Essential Medicines, 20th List, 2017.
Geneva: World Health Organization; 2017. http://apps.who.int/iris/bitstream/handle/10665/273826/EML-20-eng.pdf?ua=1
World Health Organization Model List of Essential Medicines, 19th List, 2015.
Geneva: World Health Organization; 2015. https://www.who.int/medicines/publications/essentialmedicines/EML_2015_FINAL_amended_NOV2015.pdf?ua=1



