Summary
- Even though IP has played a crucial role in developing and manufacturing vaccines for the current pandemic, some are calling for IP to be waived to facilitate preparations for future pandemics.
- This would be a mistake. In the current pandemic, IP has been central to R&D collaboration and scaling up manufacturing through often international partnerships which span every part of the vaccine value chain.
- IP is also fundamental to the ongoing innovation in manufacturing processes that has allowed global vaccine manufacturing capacity to increase multi-fold in a short period of time, producing many billions of doses.
- Waiving IP for future pandemics would disrupt these processes and leave the world reliant on open source or IP-free models of vaccine development and manufacture. Current experience with such models is not promising: they have either been extremely slow to progress through development, or have failed to mobilise sufficient capital to progress at all.
- It is not clear that the goal of appropriating IP to develop self-sufficiency in vaccine manufacturing is a sensible prioritisation of resources. A similar thing was attempted in scaling up ARV production in Africa in the 2000s, often using compulsory licenses. Such attempts failed because the resulting products were uncompetitive with those available on global markets. Other challenges include unreliable infrastructure s electricity, water, and roads, in addition to under-resourced regulators that do not have the capacity to oversee high-tech vaccine manufacturing.
- Rather than waiving IP, a more sensible approach to future pandemics is for governments to focus on supply-side approaches such as funding basic research; instituting advance market commitments; addressing trade barriers; and improving distribution capabilities.
- The protection and strengthening the R&D environment, including the robust protection of IP rights, should be at the heart of future pandemic planning to ensure the development of multiple vaccine and therapeutic options.
Introduction
For much of 2021, many low -income countries were unable to implement Covid vaccination programmes as comprehensively as most high and middle-income countries. Even though vaccine supply increased exponentially last year, COVAX, the initiative focused on delivering equitable vaccine access, only really ramped up its deliveries towards the end of 2021 due to delays to country readiness and absorption issues.
The ground laid that year in the form of massive investments and scale-up of Covid vaccine manufacturing is now paying dividends, with global supply now outstripping some countries’ capacity to deliver doses to patients. By the end of September 2021, 7bn vaccine doses were available around the world, with a total of 12bn doses manufactured by December 2021, according to health industry research group Airfinity: enough to vaccinate the world’s eligible population if evenly distributed. COVAX expects enough doses in 2022 to meet its commitments to participating countries.
Despite this promising picture, discussion continues at the WTO to waive IP to allegedly boost vaccine manufacturing and supply in low and middle-income countries. Infrastructural constraints and technical challenges mean it would be many months, if not years, from the implementation of an IP waiver before developing country manufacturing plants could start producing supply, by which time COVAX will have hit its coverage targets and the pandemic will have entered a less dangerous phase or subsided into endemic status.
Nevertheless, the debate is now shifting from the present pandemic towards preparation for future ones. A prominent claim is that developing countries will never have reliable and sustainable supplies of vaccines in the event of a future pandemic unless they can be manufactured locally, using proprietary technology and know-how forcibly appropriated – or at least aggressively impelled – from innovators if necessary. This is the backdrop to current debate at the WTO and the pandemic preparedness treaty in the early stages of discussion at the WHO.
As stated already, the global IP framework has supported the creation of a diverse and competitive market of innovative and low-cost vaccines, with product variation that gives public health planners options and choices to cater for local circumstances and clinical need. It has also supported the investment and innovation needed to scale up vaccine manufacturing to meet global demand in record time.
Removing or weakening IPRs for pandemic health technologies including vaccines would be highly counterproductive. It would undermine the incentive to invest in new technologies and treatments. Equally as important, it would disrupt the international manufacturing collaborations and partnerships that have proved so indispensable to the current pandemic. This briefing paper explores how.
IP has been the unsung hero of the pandemic
Supporters of the WTO IP waiver have been proven wrong at every step of the pandemic so far. First, there were assertions that intellectual property would hold up urgent research, with claims that the “winner-takes-all” nature of intellectual property rights, especially patents, would prevent scientists from rapidly disclosing research results.
Such fears were rapidly proven wrong by the fact that there are currently four leading Covid vaccines authorised by the most stringent regulatory authorities with 18 in late stage clinical trials or pending regulatory approval. Far from stifling information sharing, inter-organisational research collaborations blossomed in the early stages of the pandemic, with one of the most successful Covid vaccines so far owing its existence to partnership and information sharing between BioNTech and Pfizer. Such partnerships, which depend on deep sharing of large volumes of commercially sensitive material, would not occur without the legal certainty provided by IPRs.
Following the creation and market authorisation of multiple vaccines, proponents of the WTO IP waiver then claimed the suspension of IPRs was necessary to ramp up global vaccine manufacturing capacity. While manufacturing capacity did take some time to build in early 2021, the huge volumes currently being produced owe themselves in large part to IP rights.
This is because IP is fundamental to the 357 manufacturing partnerships that existed as of March 2022, often between commercial rivals. IP rights establish the trust necessary for the safe transfer of valuable manufacturing know-how without fear of it being misused for commercial gain. In the case of voluntary licensing which underpins manufacturing of the Oxford / AstraZeneca vaccine, IP rights enabled the selection of reliable and high-quality partners in multiple countries. Similarly, Pfizer and BioNTech built a network of partners for manufacturing their vaccine, which included some of Pfizer’s largest competitors. Such voluntary relationships demonstrate that IP rights provide a framework for robust and rapid technology transfer free from the reluctance, legal resistance, and natural cautions that would inevitably arise under the involuntary transfer envisioned by the WTO waiver proposal.
IP now in the crosshairs for future pandemics
With manufacturing of Covid vaccines occurring at immense volumes, all within the existing global IP frameworks, the goalposts of the debate are shifting again to the next pandemic. The claim is that Covid vaccine manufacturing is too concentrated in a handful of high-income countries, and a suspension of IP rights for Covid medical technologies is therefore necessary to enable local manufacturing of innovative vaccines in developing countries. This could allow for a more rapid deployment in case of a pandemic emergency.
This strain of thinking is being promulgated by various IP scholars and health activists to justify in the short term the IP waiver proposal under discussion at the WTO. Looking to the future, the idea that IP must be suspended to boost developing country vaccine manufacturing is informing early discussions around a possible new WHO convention or international treaty to better prepare for future pandemics (Pandemic Preparedness Treaty). Proposals being advocated include:
- Mandatory technology transfer by rights holders enforced by States, with a special enforcement role for governments in countries that house the innovative companies in question.
- Vaccine technology transfer hubs modelled on those that already exist for influenza vaccines except using compulsorily transferred proprietary technology;
- An expanded role for technology sharing platforms such as C-TAP;
- Mandatory sharing of IP via R&D funding conditionalities, for example requiring products benefitting from public subsidy to be available on an open source basis
- Funding and support for vaccine production in currently under served regions of the world.
Limitations to pandemic IP would destroy R&D incentives
Many of the proposals listed above would be extremely deleterious to pandemic research and development incentives.
IP is the bedrock upon which almost all of today’s Covid-19 vaccines have been built. The technologies they are based on did not come out of thin air at the beginning of the pandemic, but had been under development for decades, with substantial basic research in academic and government labs followed by years of risky investment by commercial start-ups to develop applications for patients.
Consider the messenger RNA (mRNA) technology which took decades of lab research and private sector-funded development by startups Moderna and BioNTech (ultimately joined by Pfizer) to overcome major difficulties and turn the technology into potentially safe and effective vaccines ready for large scale testing in patients which was fortunately very positive. Both companies and their investors spent (and risked) billions of dollars on mRNA research prior to the pandemic.
While academic research is fundamental, the end result would not have been possible without the private sector, which depends on intellectual property rights.
Shortly before the pandemic started, we spoke to Dr. Derrick Rossi, the academic founder of Moderna. When asked whether the treatments could be brought from the academic lab to patients without the help of the private sector, Dr. Rossi’s reply was categorical: “Not a chance. Academics are good at academia and fundamental science. They are not good at developing drugs for patients.”
Dr. Rossi explains that bringing a drug to market takes many professionals, sharing their labour and diverse expertise. “This industry of professionals is out there… The more people that are involved in the chain, post-academic discovery, the more you have pros involved — all the way from IP filings to VCs to due diligence to assembling a team,” the more likely you are to develop a viable treatment.
IP helps not hinders R&D collaboration
The other claim frequently heard at the beginning of the pandemic was that IP poses a barrier to collaboration and knowledge-sharing, so in a time of emergency any related IP should be open licensed or pooled, forcibly if necessary.
With Covid, the opposite occurred. The IP system encouraged the rapid establishment of dozens of partnerships around Covid-19, with even commercial rivals prepared to cooperate and share proprietary intellectual resources such as compound libraries. Some companies chose not to assert patent rights for certain Covid-related medicines and manufacturing techniques. Partnerships can involve just the private sector, but also the public sector and academia, or a combination.

IP is key to such partnerships, as it allows the safe sharing of proprietary knowledge at all stages of the drug development cycle. In the early stages, the public disclosure inherent to patent rights enabled drug developers to identify partners with the right intellectual assets such as know-how, platforms, compounds and technical expertise. Without patents most of this valuable proprietary knowledge would be kept hidden as trade secrets, making it impossible for researchers to know what is out there – something those concerned about future pandemic preparedness should bear in mind.
Additionally, the existence of laws protecting intellectual property helps rights-holders make the decision to collaborate in the first place. As Dr. Kathrin Koerner, Head of Patents & Scientific Services at Merck KGaA, explained to us in earlier research, “IP enabled the early discussions for COVID-19 collaborations and exchanges. Without it, things could not have been made available to other parties. Because we had already filed for the relevant patents, we were able to provide information to partners about things we had under development.” By allaying concerns about confidentiality, IP enables companies to open their compound libraries, and to share platform technology and know-how without worrying they are going to sacrifice their wider business objectives or lose control of their valuable assets.
For instance, rights holders might contribute IP that is useful for entirely different diseases to Covid-19 R&D. There are dozens of medicines that have been screened for efficacy against Covid or its clinical sequelae, some of which are patent protected for their original indication.
For instance, rights holders might contribute IP that is useful for entirely different diseases to Covid-19 R&D. There are dozens of medicines that have been screened for efficacy against Covid or its clinical sequelae, some of which are patent protected for their original indication.
IP is crucial to building Covid vaccine manufacturing capacity
Covid vaccine manufacturing is highly complex. There are still only a handful of facilities in the world capable of manufacturing the new mRNA vaccines, which contain several novel ingredients that required manufacturers design new manufacturing processes from scratch, scale up rapidly and build new supply chains. Meanwhile, manufacturing vaccines based on other more mature technologies is still difficult with multiple bottlenecks and production challenges.
Ingredients and input materials are still in short supply globally, such as lipid nanoparticles and mixers to make mRNA vaccines. Overriding IP rights will not increase the availability of scarce manufacturing inputs, but rather divert them from already quality-assured manufacturers to new manufacturers who will have to then go through the entire regulatory process from scratch. This will not increase the global stock of vaccines but create further delay.
Transferring technical knowledge related to vaccine manufacturing is not a simple matter of reviewing patents and other public sources. Rather, it must be taught.
Further, much vaccine production technology is not embodied by patents, but rather in technical know-how which is not easily transferred. Such information is often known by few people within the innovator organisation. Most vaccine manufacturers in developing countries lack this knowledge and without it they cannot simply or quickly repurpose their factories. Transferring this technical knowledge is not a simple matter of reviewing patents and other public sources. Rather, it must be taught.
Such transfer is indeed happening on a voluntary basis. For example, Pfizer/BioNtech and Johnson & Johnson have each partnered with their rival Merck to increase production of their cutting-edge vaccines. In fact, partnerships span the entire manufacturing value chain, existing in every continent and have been rapidly rising in number (Figure 1). Given the novelty and complexity of the technology platforms used to make Covid vaccines, these partnerships require the active transfer of technology through teaching and physical presence by key personnel. IP protections allow this to take place by giving the innovator trust and confidence that valuable information can be shared without risk.
Figure 1: Number of agreements for vaccine production at February 2022
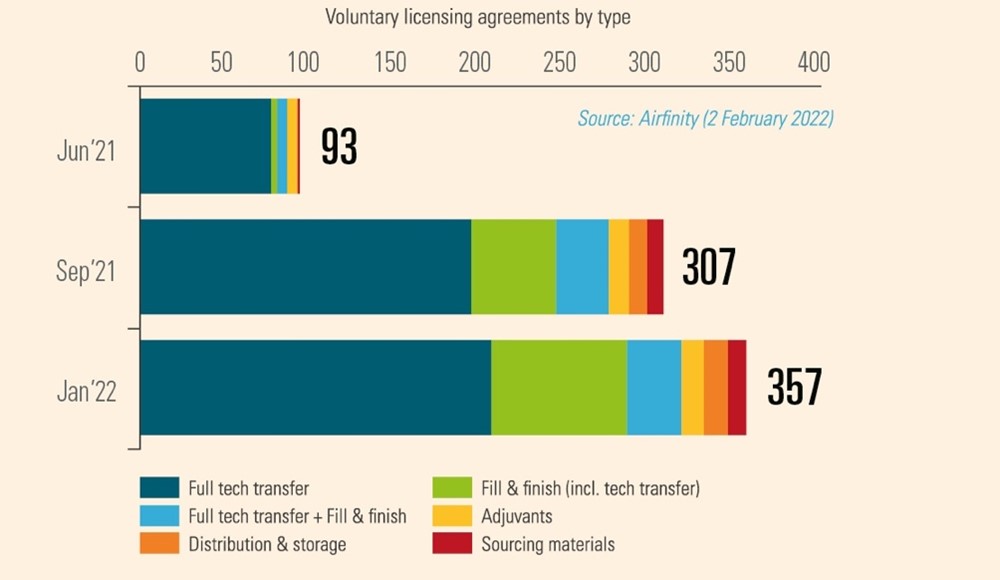
In each case, however, trade secrets and other proprietary information is protected with both agreements and the existence of laws protecting IPRs. An IP waiver or limitations of IP in a pandemic preparedness treaty would require innovators to reveal their know-how under threat of legal force, with very different consequences than voluntary cooperation.
First, forced transfers would likely be contested both in law and fact, as innovators would hardly be keen to divert their most knowledgeable and busy employees during a global crisis. Transferring this know-how could take many months, followed by further delays while regulators scrutinise any new manufacturing facilities and their products for quality standards. Pandemic-related travel restrictions would make this process even more difficult.
Moreover, forcing the disclosure of a trade secret destroys it, as it is no longer secret. Secrecy is the fundamental legal and practical requirement for the existence of a trade secret. When a patent owner is compelled to license a patent, it still owns the patent and can receive a reasonable royalty. By contrast, forced disclosure destroys a trade secret and its value.
If governments were to force technology transfer it would therefore represent a fundamental assault on private property rights and contract law which would have disastrous economic implications beyond the pandemic. At the very least it would destroy the value of that many small biopharmaceutical companies whose main assets are the IPRs they hold around small numbers of technologies.
Voluntary technology transfer based on cooperation, appropriate training and resource sharing is therefore key to establishing additional capacity.
IP drives innovation in manufacturing
In February 2022 researchers at South African biotech company Afrigen Biologics stated they had nearly made a copy of Moderna’s mRNA vaccine without infringing its patents. Although this marks a milestone for the WHO’s technology transfer hub, this is only the initial step in a long and complex road to mass mRNA vaccine manufacturing. mRNA vaccines can indeed be designed relatively quickly, albeit using knowledge and processes that took decades of trial and error and billons in investments to achieve. Moderna famously worked out the design of its vaccine over the course of two days and started human clinical trials fewer than 60 days later.
High tech vaccine manufacturing at scale requires continuing experimentation and innovation
Scaling up mRNA manufacturing to pandemic levels, by contrast, requires continuing experimentation and innovation. Just being able to produce any treatment at an experimental level is orders of magnitude short of what is needed to manufacture millions of doses. One expert described the challenge of scaling up mRNA vaccine production from the laboratory to factory with the quip: “gee, that 2000-liter reactor with process control and computers hanging off it doesn’t look much like a test tube.”
With its mRNA vaccine, Pfizer developed a 50,000 step manufacturing process, identifying and working with 86 different suppliers. The vaccine required 280 materials in total, 10 to 15 of which were novel to the mRNA vaccine. Through continuing innovation, Pfizer cut the initial production time in half, enabling it to deliver more doses more quickly, and it continues to look for opportunities to improve the process. It’s a similar story of manufacturing innovation for the Moderna vaccine.
Within the overall manufacturing value-chain, rights-holders often subcontract specific elements to specialist manufacturers. The production of plasmid DNA, crucial to mRNA vaccines, is one example. At the beginning of the pandemic manufacturing capacity was extremely limited. Since then, multiple companies have expanded capacity by innovating, each one spurred on by competitive incentives to gain market share.
One company, Touchlight Genetics Ltd, has developed an alternative to plasmid DNA – “doggybone” DNA (dbDNA). It can be produced in weeks rather than the months required for plasmid DNA, and Touchstone states that it can produce enough for 1 billion vaccine doses a month. While the technology itself is patented, Touchlight has worked for several years to optimise its manufacturing techniques. In 2018, Touchlight partnered with Janssen Biotech to evaluate and refine its production processes for its patented technology.
These sorts of collaborations, requiring technology transfer, as well as the development and sharing of valuable proprietary information, depend on the trust engendered by trade secret laws and other forms of IP protection.
An IP waiver, or limitations on IP for future pandemics, would disrupt this process of market-based discovery and innovation. Even if existing manufacturing technology were to be transferred to plants in developing countries, incentives to refine and innovate manufacturing processes would be significantly diminished. Given that future pandemics will almost certainly require different technologies to those currently being deployed, it is crucial to retain the incentive system that has worked for this pandemic.
Alternative vaccine development models have yet to deliver
As part of this debate, some have suggested that the current IP-based model of drug and vaccine development could be replaced in pandemics with open source and other IP-free models. Such models were already available to researchers and governments at the beginning of the pandemic, and now in early 2022 enough time has passed to make an initial assessment of their effectiveness.
In summary, while a few examples have advanced towards market authorisation, their development has been too slow for a pandemic situation. This is largely due to open source / IP free models struggling to attract the necessary funding to advance rapidly through clinical trials and establish large levels of manufacturing capacity (See box).
IP-free Covid vaccines struggle to advance
University of Helsinki Vaccine
In May 2020, researchers from the University of Helsinki claimed to have developed a Covid vaccine nasal spray that they wanted to be “the Linux of vaccines”, free from any patents or other forms of intellectual property protection. The researchers received a public grant of over a million Euros to develop it, announcing in March 2021 that preclinical tests had been successful and the candidate vaccine was ready to advance to larger clinical trials. The team failed to secure the necessary funding for clinical trials, and there are currently no details about the preclinical studies available publicly.
Corbevax
Corbevax is a protein subunit Covid-19 vaccine developed by Texas Children’s Hospital Center for Vaccine Development and Baylor College of Medicine in Houston, Texas and Dynavax technologies. Corbevax is available as a patent free vaccine and its developers have no financial stake. Unlike the Finnish vaccine, the developers of Corbevax did manage to secure funding to help it through clinical trials, from sources including CEPI and India’s Biotechnology Industry Research Assistance Council. Although it has been authorised for use in India, neither Corbevax’ developers nor the Indian regulatory authorities have released any data on the vaccine’s efficacy and none on any clinical trial. It is therefore impossible to comment on its usefulness.
What is clear is that Corbevax has had a far slower development timeline than its rival for-profit / IP-dependent vaccines, and has so far failed to secure authorisation from a stringent regulatory authority.
If successful, the IP-free Covid vaccines described in the box could prove useful additions to the Covid vaccine arsenal. But it is unlikely that the difficulties such patent-free models have faced in marshalling the large amounts of capital and expertise necessary to rapidly scale-up global production mean they are unlikely to make a global difference, at least for this pandemic.
By contrast, vaccines that have leveraged IP rights have moved quickly through clinical development and into mass manufacture and distribution. Within this framework, different models and approaches have emerged: for example Pfizer/BioNTech developing a global network of contract manufacturers and distributors spanning four continents and over 20 facilities; and the model pursued by Oxford /Astra Zeneca in which manufacture is out-licensed in its entirety to partner manufacturers in different countries. The existence of the IP system allows for this experimentation and diversity.
That is not to say such open source or patent-free initiatives should be discouraged. As much effort as possible should be directed at the problem of developing and manufacturing vaccines for pandemics. But removing IP either now in the form of the WTO TRIPS waiver or in future via a Pandemic Preparedness Treaty would likely disincentivise the private sector to the extent of non-participation.
Relying on open source and IP-free vaccine development approaches would be highly risky in a pandemic situation
The world would then be forced to rely on the open source / patent-free approach, which is highly risky given its patchy track record. It would be far better to maintain current incentives to ensure a diversity of approaches and the involvement of as many categories of stakeholder as possible.
Towards vaccine manufacturing capacity in developing countries
Vaccine manufacturing plants cannot just be parachuted into developing countries and be expected to run effectively and economically. They require at a minimum supporting physical infrastructure, in the form of reliable electricity, clean water supplies and access to transport infrastructure including international air freight. Reliable electricity and water supplies cannot always be guaranteed even in South Africa, which has suffered from electricity load shedding and water rationing in recent years.
Vaccine manufacturing also requires highly skilled employees, who may be tempted to seek better remuneration and life opportunities overseas. “Very often what we see is that newly upskilled employees seek opportunities elsewhere very often outside of the [African] continent,” Patrick van der Loo, regional president for Africa and the Middle East at Pfizer, told a stakeholder meeting on vaccine manufacturing of the Africa Centre for Disease Control and Prevention. Martin Friede, coordinator of the World Health Organisation Initiative for Vaccine Research, told Reuters that Africa had produced many scientific researchers but not a workforce capable of designing and making vaccines.
The successful introduction of a new vaccine manufacturing plant also requires a strong local regulator who can ensure facilities meet the necessary safety requirements. These are often lacking. However, no National Regulatory Medicines Authorities have achieved the World Health Organization’s (WHO) maturity level 3, considered a prerequisite to eventual WHO prequalification of local vaccines.
In addition, delays and backlogs average between four to seven years between first regulatory submission to a well-resourced National Medicines Regulatory Authority and final approval in Sub Saharan Africa.
A more positive way to attract investment in vaccine manufacturing is to address these issues. Without progress here, waiving or limiting intellectual property rights will achieve little, other than discouraging investment from the innovative vaccine industry.
Lessons from local manufacturing for HIV ARVs medicines
Moves to limit IP for pandemic technologies are somewhat based on the premise that less developed countries in Africa and elsewhere should become self-sufficient in vaccine manufacturing. This objective has won support from major global health stakeholders including the IMF, the Africa Centres for Disease Control and Prevention (ACDC), GAVI and others.
ACDC in particular has proposed a pan-African hub and spoke manufacturing model in which vaccine sub-components are manufactured by different countries, with support from multilateral agencies such as Gavi to act as a “market shaper” by guaranteeing procurement.
There are parallels with attempts by multilateral and overseas development agencies to establish anti-retroviral (“ARV”) manufacturing capacity in Africa in the 2000s. Then, the aim was also to secure sustainable access to medicines and increase technical and industrial capacity of countries. The Pharmaceutical Manufacturing Plan for Africa, rolled out in 2005 by the African Union Development Agency, created a business plan to boost local pharmaceutical production and improve public health outcomes.
In terms of intellectual property rights, several countries in sub Saharan Africa invoked flexibilities in the TRIPS Agreement to manufacture locally patented ARVs under compulsory license, including Ghana, Zambia, Mozambique and Zimbabwe.
In 2005 the World Bank produced a summary of the evidence surrounding local production of pharmaceuticals, concluding that producing medicines domestically in many parts of the world makes little economic sense: “If many countries begin local production, the result may be less access to medicines, since economies of scale may be lost if there are production facilities in many countries,” the authors concluded.

Julphar.uae, CC BY-SA 4.0 via Wikimedia Commons
The evidence since gathered bears this out. Researchers have been able to find little evidence that local production strategies facilitate access to medicines, nor that it achieves the other benefits claimed by its proponents, such as foreign import savings, enhanced human capital and greater local innovative capacity. Other researchers looking at specific case studies have found some initial successes in establishing facilities in Africa, but little answer to the question of how they will be sustainable in the absence of substantial foreign financial assistance.
For pharmaceutical and indeed vaccine manufacturing, the key concept is comparative advantage, specifically how to make pandemic vaccines manufactured and developed in LDCs in Africa and elsewhere competitive with those produced elsewhere. Again, the HIV pandemic provides a useful lesson: one major review of ARVs locally manufactured under compulsory license in Africa were found to be substantially more expensive than those procured on global markets through the Global Fund to Fight AIDS, Tuberculosis, and Malaria, UNICEF and other international channels.
In the cases of Zimbabwe, Zambia and Mozambique, local manufacturers working under compulsory license found themselves eventually displaced by Indian generic manufacturers, who are able to produce medicines more cheaply. The cost of imported APIs is also a major constraint, and doubly so for mRNA vaccines which rely on hundreds of imported subcomponents and manufacturing parts and supplies.
What can be done?
The foregoing shows that IP has played a crucial role in addressing the current pandemic and will be key to future pandemics. But as we offer reasons to be sceptical of pandemic preparedness proposals that focus on limiting or appropriating IP and reducing the role of the private sector, one might ask whether governments must simply wait and hope for the private sector to rescue their people. The answer is “no,” and the fight against Covid-19 offers several lessons as to how governments can play a crucial role in partnership with the innovative pharmaceutical sector to facilitate the development, manufacturing, and distribution of vaccines.
Fund basic research
First, governments can fund basic research into vaccines and treatments. Governments, universities, and non-profits play a crucial role in funding basic research. For example, from the discovery of mRNA in the early 1960s, to key advances such as the use of lipid nanoparticles to deliver mRNA into the human body, to determining how to mute the inflammatory response to mRNA, government grants and non-profit research institutes were essential to developing the foundations of mRNA vaccine technology. The private sector later took the risks and raised the billions of dollars necessary to develop applications of these building blocks.
This division of labour, where governments fund the lion’s share of basic research and the private sector role increases as necessary investments, risks of failure, and costs increase with developing practical treatments, is well-tested and successful. The prioritisation of pandemic preparedness in government funding will ensure progress continues.
Pool advance purchase commitments
Second, governments can use procurement contracts to incentivise vaccine development. When Covid-19 emerged, the US., UK, EU, Canada and other governments made purchase commitments to several companies with potential vaccine candidates. A key feature of these agreements was the commitment to purchase a large quantity of doses if the vaccine was successful, in addition to strong policy support for R&D in non-pandemic times.
These purchase commitments spurred vaccine development, as they removed some of the risks. The risk of failure was still present – for example, Merck took a large charge in the fourth quarter of 2020 for its investment in a failed Covid vaccine candidate. However, several other risks were mitigated, such as that another vaccine candidate might be first to market. Even today, in early 2022, there are commitments to purchase certain vaccines still pending – for example, the Novavax protein subunit vaccine may soon be approved for use. Moreover, other risks include that another successful vaccine might be preferred due to slight differences in efficacy, advantages in delivery, or even politics.
Mitigating these risks with purchase commitments has helped ensure a diverse and robust supply of vaccines. Companies were able to invest not only in development, but in the unprecedented early development of manufacturing capacity and production of doses, even before approval.
As much as these commitments were a boon to development and delivery, however, they proved to be somewhat of a problem when it came to equitable distribution of vaccines. Manufacturers were contractually bound to put the countries that had contracted early first in line. This resulted in some countries having a surplus of doses and the ability to vaccinate and even boost their less vulnerable populations while others still waited to begin vaccinating their most vulnerable.
In the future, governments and international organisations can develop mechanisms to pool resources to get the benefits of purchase commitments with less of the downside of vaccine nationalism. The COVAX initiative’s premise that governments should pool resources to distribute vaccines widely was sound. However, the pool will work more equitably and effectively if it has a broader membership and is better resourced,
Remove trade and regulatory barriers
Vaccine production is a widely distributed, multinational effort with globally distributed supply chains. The leading vaccine producers worked with a network of suppliers and manufacturing partners that spanned the globe and every continent. At times, however, this global supply chain was when governments imposed trade barriers to try to reserve access to key supplies. When supply chains are global and distributed, such disruptions hurt everyone, including those who impose them.
One of the most prominent examples of a trade barrier was India’s ban on vaccine exports that lasted for many months. India, which has long prided itself on being the “pharmacy of the developing world” was slated to play an essential role in fulfilling global demand and supporting the COVAX initiative. The export ban thwarted these expectations for many months.

Governments must credibly and effectively commit to keeping global supply chains for vaccines and disease treatments open during health crises. Such a commitment requires more than a mere resolution or promise, but, rather, the sort of trade commitments with enforcement mechanisms that are the foundation of the World Trade Organisation.
Moreover, governments need to develop greater regulatory flexibility, agility, and harmonization. There were laudable and necessary efforts in this regard, as governments found ways to speed approvals of vaccines and other treatments. These examples should be improved upon, institutionalized, and adopted universally.
Greater regulatory harmonization would also play an important role. It was often the case that once vaccines were placed in vials and labelled for use in one country, they could not be redeployed to another, due to differing regulatory requirements. If approvals, specifications, and labelling could be harmonized then such inefficiencies could be avoided.
Improve distribution capabilities
As vaccine supply constraints have eased, another issue has emerged. Even as manufacturers deliver vaccines, some countries and public health systems are struggling to ensure that these vaccines reach patients. Infrastructure, organization, personnel, processes, and other resources don’t always exist to bring the vaccine the last 100km it needs to go to reach a patient. According to Seth Berkley, CEO of the Gavi vaccine alliance and co-ordinator of COVAX, “the challenges range from shortages of supplies like syringes or health care workers to utilize them, issues with the ‘cold chain’ needed to transport and store vaccine doses, vaccine hesitancy, or inadequate logistical planning.”
Governments and NGOs can play a key role in planning, organisation and greater investment in such deploying such resources, including investing in cold chains, increasing the number of vaccinators, and supporting efforts to tackle supply chain and logistic bottlenecks.



