The Covid pandemic has energised opponents of the WTO TRIPS Agreement, who have long wished to weaken permanently intellectual property (IP) rules.
But opposition to TRIPS ignores the huge benefits brought to WTO members by enabling increased participation in the global economy and facilitating access to new technologies – not least the creation and production of innovative Covid vaccines and therapeutics in record time.
Meanwhile, the IP flexibilities reaffirmed by the Doha Declaration, signed 20 years ago this month, have proven an effective public health safeguard. In fact, such flexibilities have rarely been invoked due to the success of various mechanisms that deliver innovative medicines to low and middle-income countries within the existing TRIPS IP framework, including voluntary licenses and initiatives such as the Medicines Patent Pool.
As WTO members gather in Geneva for their 12th Ministerial Conference, it’s time to reject shop-worn criticism of TRIPS and IP, and recognise instead their tremendous contribution to global health progress.
Introduction
The Covid pandemic has underscored the importance of the TRIPS Agreement, the WTO-administered treaty that provides basic global standards for intellectual property (IP) rules. The global framework of legally-enforceable IP rules provided by TRIPS has become an essential part of the global economic plumbing, as knowledge-based goods and services have come to dominate international trade over the last 25 years since the Agreement’s ratification.
Nevertheless, TRIPS has an undeservedly bad reputation. Perceptions of TRIPS have been warped by a near-constant stream of ideological anti-IP academic articles, hostile Geneva panel discussions and documents issued by UN agencies that promulgate the view that TRIPS is a developmental disaster for low and middle-income countries, a zero-sum deal that was forced on them during the GATT negotiations as a kind of Faustian bargain to secure market access to the US and EU.

As time has passed, this view of the TRIPS Agreement is looking increasingly dated. As the global economy has shifted over the last twenty years from low-value physical manufacturing to the creation and trade in knowledge-based goods and services, the level international IP playing field introduced by TRIPS has enabled many developing countries to participate more meaningfully in the global economy. Participation in knowledge-intensive global value chains and innovation networks has delivered transformative improvements in living standards and development indicators in many parts of the world.
Meanwhile, basic global standards of IP rules under TRIPS has allowed modern health technologies to spread more rapidly around the world, improving both access and incentives for innovation. The Doha Declaration of 2001 reaffirmed the right of WTO members to have certain flexibilities in circumventing patent rights for access to essential medicines in pressing situations. Although as this paper will later point out, WTO members have only rarely felt the need to invoke TRIPS flexibilities due to successes of approaches that work within the existing IP framework, most notably voluntary licenses.
And the most notable health crisis of recent years – the Covid-19 pandemic – is being addressed by innovations that have taken place thanks to IP. Contrary to popular belief, both innovation and production of innovative Covid vaccines has depended on international collaboration, facilitated by the international IP rules enshrined in the TRIPS Agreement.
For opponents of TRIPS, though, the Covid pandemic represents a major opportunity to reset global IP rules relating to health. In the autumn of 2020, before any new vaccines were available, India and South Africa put a proposal before the WTO to allow a temporary suspension of certain IP commitments within the TRIPS Agreement for Covid-19-related technologies, arguing that IPRs would be a hindrance to mass manufacturing and global roll-out of new Covid vaccines.
While the WTO has yet to reach consensus on this proposal, the scope and depth of the IP waiver advocated by its sponsors represents a great challenge to the TRIPS Agreement and the Doha Declaration of 20 years ago. It would destroy the economic value of some of the most promising health technologies of recent times, and set a precedent that would make it politically far easier to suspend IP rules in the future.
Unpicking the TRIPS Agreement via the WTO waiver would be a serious mistake, threatening progress in health, innovation and development. This policy brief sets out the benefits of the TRIPS Agreement and outlines the progress that will be put at risk by the proposed TRIPS Waiver.
Unpicking the TRIPS Agreement via the WTO waiver would be a serious mistake, threatening progress in health, innovation and development
TRIPS and economic, health progress
Much of the negative criticism of the TRIPS Agreement fails to take account of the remarkable progress that has taken place in developing countries since its ratification in 1994. Over the last 25 years, despite some bumps in the road, economic growth has accelerated and billions have been lifted from poverty. Partly thanks to this economic growth global health has been getting better, with key metrics pointing to significant and sustained improvements almost everywhere since the ratification of TRIPS (Figures 1 & 2).
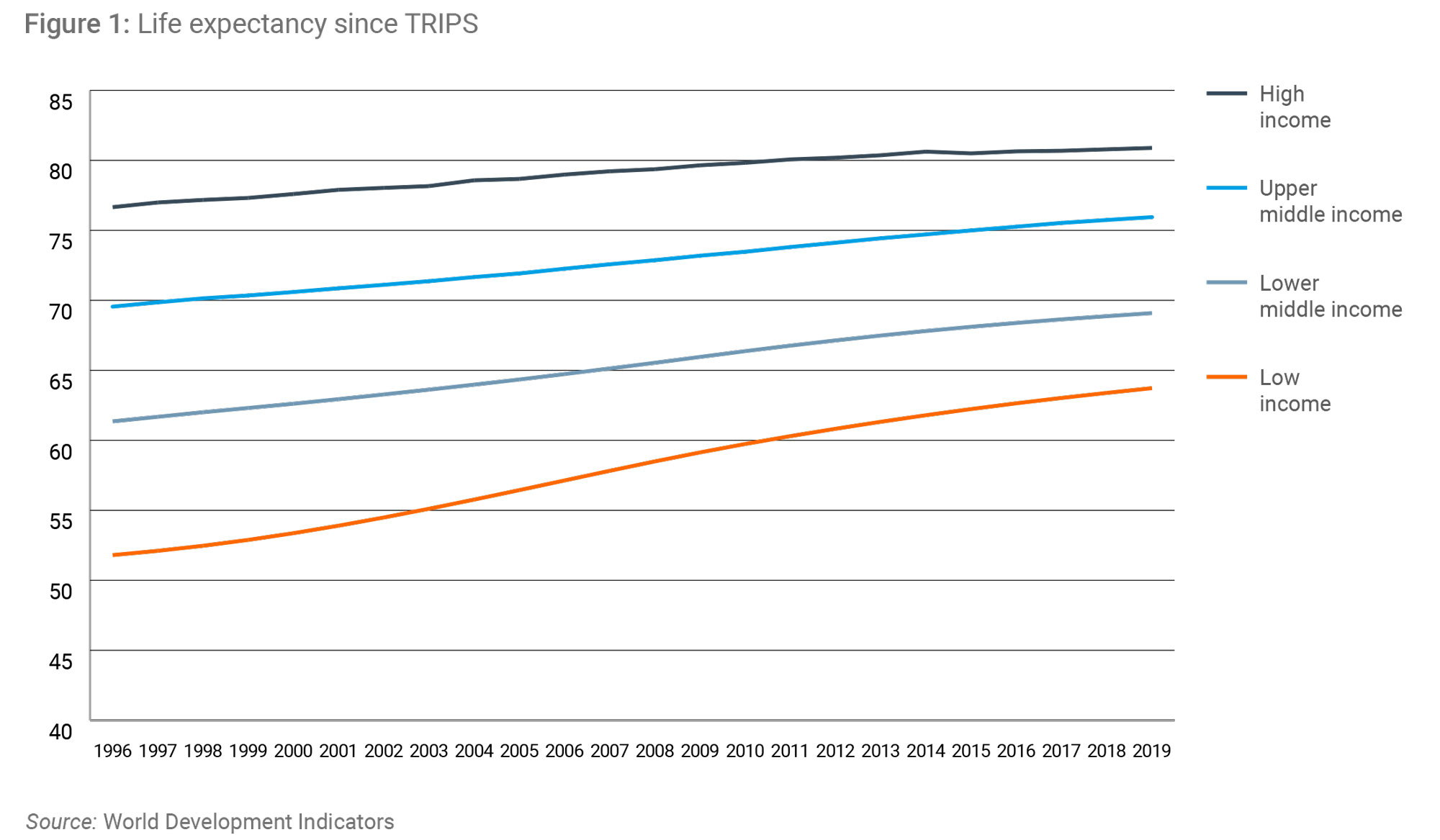
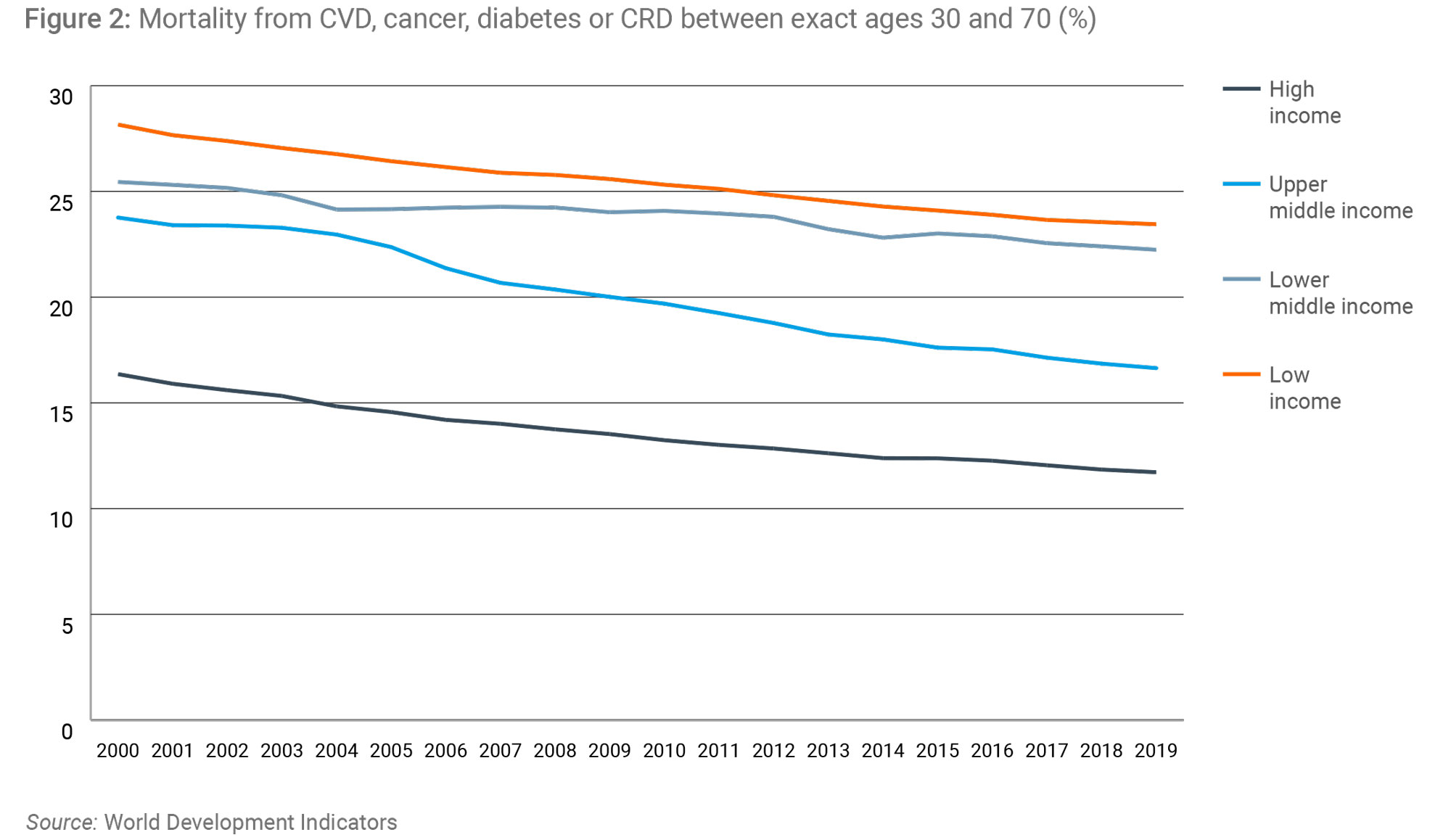
Why global IP rules matter
The economic and developmental progress made by most middle- and low-income countries since the ratification of TRIPS illustrates the reductiveness of viewing the agreement solely through the narrow prism of medicine patents. In the early 1990s, it was becoming clear that in an increasingly knowledge-based global economy, there was a need for an overarching multilateral agreement to replace the ineffective pre-existing patchwork of deals that protected IP. Disagreements over IPR were acting as a significant non-tariff barrier to trade, chilling investments, technology transfer and licensing and the diffusion of technological goods to the developing world.
A global framework to govern intellectual property rights has become even more important over the last twenty years as global trade has become less about moving physical goods from their point of manufacture to customers in different countries, and more about trade in “intangible” products and services, based on research and development efforts, brands, and patented or licensed technology.
Global cross-border exports of commercial knowledge- and technology-intensive goods and services reached an estimated $4 trillion in 2014. Knowledge-related input represents about one-half of current global trade flows, growing at about 1.3 times the rate of labour-related flows.

Low and middle-income countries have become more connected to this trade not only through imports of finished goods, but also through increased participation in Global Value Chains, in which goods are designed and manufactured across many different countries. Increasing economic value within these value chains is attributable to knowledge-based capital such as R&D, design and branding, (Figure 3) contrasting with older times when most value was derived from manufacturing itself.
According to research from WIPO, income from intangibles in major manufacturing industries increased by 75 percent from 2000 to 2014 in real terms, totalling USD5.9 trillion in 2014, much of which has accrued to Low and Middle Income Countries. According to World Bank research, participation in Global Value Chains has sharply reduced poverty in those countries that have integrated most deeply into them, including Bangladesh, Vietnam and China.
Middle-income countries have also become more deeply involved in Global Innovation Networks, in which companies disperse their R&D activities across countries, both with overseas subsidiaries and outside partners. Asia is a particular beneficiary of this trend, with data showing a high proportion of co-invention with foreign partners in patents filed in the region (the highest number being in Malaysia and Singapore).
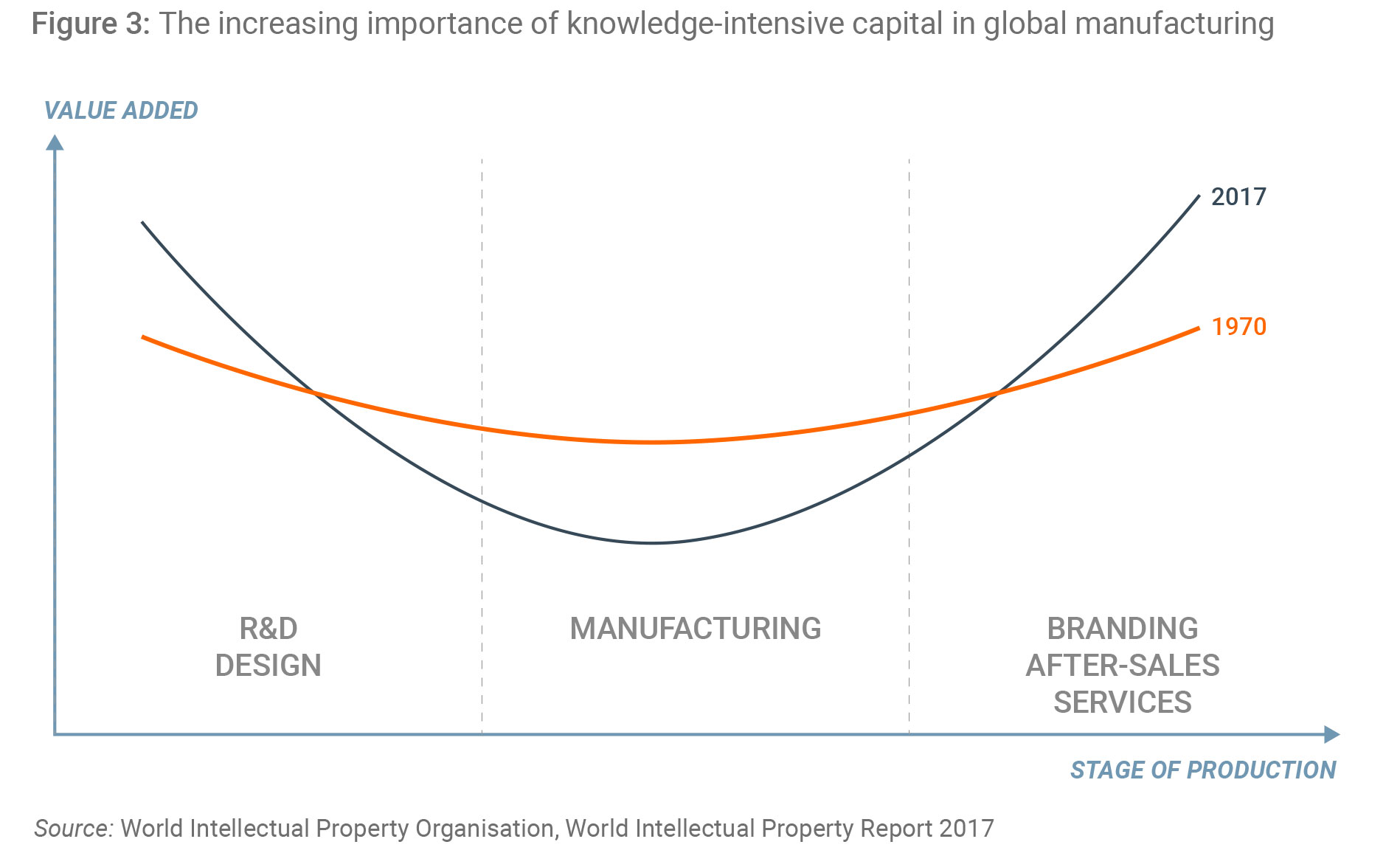
Without the IP rules overseen by TRIPS, it is unlikely these value chains would include LMICs, where IP protection was previously uncertain or non-existent. Knowledge-intensive companies that oversee these global value chains would be highly unlikely to contract with partners in other countries in the absence of robust IP rules. The legal certainty provided by TRIPS therefore makes these value chains possible, to the economic benefit of millions of people in low- and middle-income countries how have found new sources of higher-value employment. If TRIPS were unravelled, those countries that chose to ignore or weaken IP would be left behind as the global economy moved on.
Without the IP rules overseen by TRIPS, it is unlikely that global value chains would include low- and middle-income countries, where IP protection was previously uncertain or non-existent.
Technology transfer and local innovation.
Academic evidence shows that the existence of IPRs is a pre-requisite for technology transfer, which in itself is necessary for local industries to upgrade their own technological capacity and productivity. Stronger patent protection is particularly linked to increased technology transfer to developing countries via the transfer of technology-intensive goods, services and capital. This is important because the transfer of technology by foreign firms can contribute up to 90% of domestic industrial productivity, which ultimately boosts economic growth.
Empirical studies also show that strong patent protection in any given country in the longer term promotes indigenous innovation by local companies, suggesting that the IP rules established via TRIPS have had beneficial local impacts. The Indian biopharmaceutical industry is an interesting example of this phenomenon (box).
TRIPS brings innovation to Indian pharma
The Indian pharmaceutical industry is a good case study how the institutional change ushered in by TRIPS has spurred its local industries into becoming more innovative. Following the 2005 Patent Amendment Act, IP standards were considerably increased in India. Research shows that the innovation outputs (such as numbers of patent filings and international partnerships) of Indian biopharmaceutical companies sharply increased in the years directly after this major institutional change.
This evidence shows TRIPS has encouraged Indian companies to transition from imitation-based to innovation-based business models. Those Indian firms entering into cross-border partnerships with foreign companies have proven the most successful at enhancing their innovative capacities thanks to the international transfer of knowledge, skills and know-how. These international partnerships were impossible for Indian companies before the IP standards brought in by TRIPS.
TRIPS and access to medicines
What of complaints that stronger IP protection at the national level, stemming from the implementation of TRIPS, has undermined access to medicines? Here the oft-heard argument is that the temporary period of market exclusivity conferred by patents allows companies to raise medicine prices to supra-normal levels, putting medicines out of the reach of most patients, particularly those in developing countries where healthcare is typically paid for out-of-pocket.
Evidence suggests this scenario, while theoretically plausible, doesn’t generally play out in the real world. India once again provides a useful case study, as the introduction of national legislation in 2005 to comply with the TRIPS Agreement provides a clear dividing line between and pre and post IP environment. In studying the impact of TRIPS on medicine prices in India, economists
Looking globally, Margaret Kyle and Qian Yuan found pricing premium associated with existence of patents “close to zero, on average, in the lower-middle income countries”, although patented drugs sell at a higher premium in developed countries.
While the impact of TRIPS on medicine prices in developing countries is marginal, the impact of stronger IP protection on the availability of medicines is clearer. Borrell (2005), examining HIV treatments, found that patents were associated with faster launch in developing countries with relatively low levels of income inequality. Kyle and Qian (2013) found that the existence of patents is associated with faster drug launch and higher sales, while Cockburn, Lanjouw and Shankerman (2015) found that longer and more extensive patent protection accelerates drug launch in any given country. In India, Berndt and Cockburn (2014) found significant launch delays in India due to weak patent protection, with 50% of new drugs launched in India 5 years after worldwide launch.
While the potential for the patent protection brought in by TRIPS to limit access is often emphasised in policy discussions, this evidence shows that the existence of IPRs in fact increases the availability of new treatments to populations in developing countries. This is likely due to the increased incentives of investing in drug launch, including marketing, regulatory procedures and building distribution infrastructure. No company would make these significant investments in new drug launch if IP protection were absent, investments that pave the way for subsequent generic entry.
The Doha Declaration at 20 years: working as it should
The other facet of the TRIPS access to medicines debate is the impact and scope of 2001’s Doha Declaration, which reaffirmed the IP flexibilities available to WTO Members to improve access to medicines. In particular, years have been spent debating and ultimately amending Paragraph 6 of the Doha Declaration, which focuses on the right of Members without domestic manufacturing capacity to import compulsory licensed medicines from abroad. This debate at the WTO ultimately led to the first (and to date only) amendment to TRIPs in the form Article 31bis, coming into force in 2017.
Compulsory licensing for export inspired by Paragraph 6 of the Doha Declaration has only taken place once (in 2007 between Rwanda and Canada), despite the establishment of Article 31bis. A number of WTO Members, academics, and NGOs have argued that the limited use of the system shows that there should be further flexibilities within TRIPS, and that the system works against public health interests.
The claim that the limited use of the Paragraph 6 means the Doha Declaration is a failure does not stand up to scrutiny. As international trade expert Eric Solovy has recently argued, Paragraph 6 has only been invoked in exceptional circumstances, as intended by the system.
The limited use of the TRIPS Paragraph 6 system does not mean it is a failure. Rather there are multiple other more sustainable options to increase medicine access while respecting IP rules
In fact, as has been pointed out by multiple WTO Members, the system has rarely been invoked due to multiple options within the existing TRIPS framework that have tangibly improved access to medicines. These include the emergence of a global architecture of health funding and procurement intergovernmental organisations, and the widespread use of voluntary licenses for generic manufacture (seen most notably for patented HIV and Hepatitis C drugs). The poorest countries are excluded from many pharmaceutical-related obligations under TRIPS as part of their transition period, meaning drug patents are often not registered or enforced. Many companies choose to sign “non-assert declarations”, meaning they will not pursue patent protection in certain developing countries.
The fact that both Pfizer and Merck have recently announced licenses for their innovative Covid-19 therapeutics to allow for manufacture through the UN-backed Medicines Patent Pool further demonstrates how access to innovative medicines for LDCs can be achieved within the existing TRIPS framework.
TRIPS has delivered for Covid-19
In fact, the existence of a global legally-binding IP framework under TRIPS has been fundamental to the success of both R&D and manufacturing scale-up of Covid-19 vaccines therapeutics. Consider that in early 2020 Covid-19 went from being a relatively unknown disease. By October 2021 there were four available vaccines authorised by western regulatory authorities, and a further 18 available from China, Russia, India and elsewhere. 91 vaccines were at various stages of clinical trials at the time of writing.
This innovation success has been matched by previously unthinkable increases in vaccine production capacity. By the end of September 2021, 7bn vaccine doses were available around the world, with a total 12bn doses expected to be manufactured by December 2021, according to health industry research group Airfinity: enough to vaccinate the world’s population.

Much of this success is due to the legal certainty around IP rights provided under TRIPS, which has promoted trust, knowledge-sharing and collaboration between individuals and organisations. A global level playing-field of IP rules has enabled dozens of research collaborations and manufacturing partnerships all over the world, often between competitors. Rivals have shared proprietary compounds, platforms and technologies to develop new vaccines in record times. Vaccine developers have joined forces across borders with manufacturers – many of them commercial competitors – to boost manufacturing capacity to an unprecedented level.
By allaying concerns about confidentiality, IP enables companies to open up their compound libraries, and to share platform technology and know-how without worrying about losing control of their valuable assets.
How TRIPS promotes Research and Development
Far from being a barrier to sharing knowledge, as critics of TRIPS have claimed, IPRs have been fundamental to research collaboration and innovation. Because patent rights require public disclosure, they enable drug developers to identify partners with the right intellectual assets such as know-how, platforms, compounds and technical expertise. In a pandemic situation, where speed is essential, this public disclosure of patents was no doubt a major factor behind the speed with which research consortia and collaborations established themselves at the beginning of the pandemic, many of them cross-border.
Second, the existence of laws protecting intellectual property helps rights-holders make the decision to collaborate in the first place. By allaying concerns about confidentiality, IP enables companies to open up their compound libraries, and to share platform technology and know-how without worrying they are going to sacrifice their wider business objectives or lose control of their valuable assets.
For instance, rights holders might contribute IP that is useful for entirely different diseases to Covid-19 collaborations. IP rights and licensing ensure those rights can only be used for the agreed reason, preventing competitors freeriding to gain an unfair advantage in other areas.
Examples of consortia between the private sector and research centres include the Covid-19 Therapeutics Accelerator to evaluate new and repurposed drugs and biologics, the EU-backed Swift COronavirus therapeutics REsponse, Corona Accelerated R&D in Europe (CARE) as well as dozens of bilateral agreements between companies. Famously, the Pfizer vaccine is the result of its collaboration with BioNtech, where partners shared and combined know-how and proprietary knowledge to create the first vaccine authorized in the U.S.
Suspending the TRIPS Agreement would severely damage Covid-19 R&D, by discouraging the private sector from investing in vaccines for new variants or improving vaccine storage and delivery. It will also hurt preparations for future pandemic preparedness by dissuading companies from sharing their proprietary knowledge with researchers and partners.
Manufacturing
Global manufacturing of Covid-19 vaccines now stands at around 1.5bn doses per month and growing. Prior to the pandemic large corporations like Pfizer and J&J had some production capacity, but far below the levels needed to deal with a global problem like Covid. To increase manufacturing capacity, such companies turned not only to contract manufacturers but also to competitors. Given the novelty and complexity of the technology platforms used to make Covid vaccines, these manufacturers had to be taught how to make the vaccines. IP protections allowed this to take place by giving the innovator trust and confidence that valuable information could be shared without risk.
As an example, a Wall Street Journal article of August 2021 documents the volume and complexity of proprietary information that Pfizer shares with manufacturing partners. In establishing a manufacturing partnership with specialist health manufacturer Thermo Fisher, for instance, Pfizer disclosed “more than 500 top-secret files – at least 5,000 pages of documents on making the vaccine – over secure computer servers and trained Thermo Fisher workers on mRNA, which the plant had never used before.”
Following the assembly of vaccine components, specialist “fill and finish” companies then place the vaccine into vials. More know-how must be shared and transferred to accomplish this. The Wall Street Journal reported that “just transferring the knowledge of filling and capping the vials typically takes about 18 months and involves 10 stages, each consisting of hundreds of steps during which dozens of things can go wrong.”
Without IP protection collaboration and transfer of know-how and trade secrets could not have occurred, not least as the technology behind the mRNA vaccines has many other commercially valuable non-Covid applications. According to Bryan Zielinski, Chief Patent Counsel at Pfizer, “the same way that BioNTech was able to work with Pfizer due to IP protection, we were able to work with partners on manufacturing deals. Patents provided security, in addition to know-how and trade secret protections.”
The case study documented above took place in the United States, where standards of IP protection are high. But Covid vaccine manufacturing partnerships exist all over the world, in middle-income countries such as South Africa, Brazil, India, China, Mexico and Argentina. These international partnerships would be unlikely to have emerged in the absence of global IP framework provided by TRIPS.
Vaccine distribution: the most pressing public health challenge
With Covid-19, the world is on course to manufacture enough vaccines, yet access remains unequal globally. This suggest issues other than IP are responsible for the unequal global roll-out, many of which are political or related to long-standing weaknesses in public health infrastructure in developing countries. Yet a large proportion of the global debate is focused on IP.
This has echoes of the time leading up to the Doha Declaration, in which a large proportion of the public discourse was consumed by arguments over pharmaceutical patents in general and TRIPS in particular. But as Pascal Lamy, the European Commissioner for Trade at the time of the declaration, put it, Doha “solved about 10 percent of the problem of access to medicines by developing countries.”
In the late 1990s and early 2000s, a lack of health infrastructure in low and middle-income countries was a key determinant of poor rates of access to HIV and other essential medicines, something the Doha Declaration did nothing to address.
For Covid, attempting to build new manufacturing capacity by waiving TRIPS – even if it were feasible – does not seem to be the most pressing public health priority. Rather, the focus should be on logistics and ensuring vaccines can move safely from airports into the arms of people in low and middle-income countries.
There are actions governments can take both at the national and international level. Production of vaccines has been impacted by export restrictions and tariffs on imposed by many countries. Tariffs on critical vaccine manufacturing inputs reach up to an average 10%, according to the WTO.
Meanwhile, regulatory bottlenecks can lead to delays in granting market authorisation for new Covid vaccines. For example, Japan’s vaccination programme for a long time lagged behind peer countries in part due to regulatory delays in approving new mRNA vaccines for use in the country. India was ill-placed to address the Delta wave early in 2021 due to shortages of vaccines, yet at the time it had yet to approve mRNA vaccines for use.

These issues are addressable through targeted policy measures, with WHO, WTO and other multilateral agencies well-placed to play key roles in monitoring and reducing trade and regulatory barriers. Indeed, various proposals from Members are currently before the WTO that hope to address these issues, such as the Trade for Health Initiative (TAHI) put forward by the Ottawa Group. Yet the high-profile of the IP debate at the WTO sucks political energy away from these pressing issues, to no real purpose.
Conclusion
In today’s globalised world, a new invention is rarely the product of one company in one country. R&D networks are increasingly dispersed across borders, with participation from all kinds of private and public sector organisations. This is the most efficient way of tapping into the world’s brainpower and know-how, yet it would be impossible without TRIPS.
Without TRIPS, large companies would continue to innovate and manufacture, but would increasingly take things in-house and draw back from international partnerships and investment. This less efficient “vertically integrated” way of working would ultimately lead to higher drug development costs, slower R&D and ultimately fewer, more expensive innovations. In a world where the international protection of IP is uncertain, smaller companies would be unable to collaborate in R&D and manufacturing, and those in particular from countries with weak IP would fall by the wayside. Disadvantaging fledgling local companies in this way would make the transition to high-income status much harder for such countries.
By setting the precedent that the TRIPS agreement can be disregarded, the proposed TRIPS waiver would spell the end of the agreement as a meaningful framework for IP protection. This would be a huge own goal.
In the case of Covid, TRIPS has enabled a plethora of international collaborations in R&D and manufacturing that has put the world in a strong position to end the pandemic, in which valuable proprietary information has been shared with all kinds of partners including competitors. This collaboration would have been impossible without the legal certainties provided by the TRIPS Agreement.
That is not to say TRIPS is perfect. TRIPS sets only basic standards of IP protection; it was negotiated in the late 1980s and early 1990s when the Internet barely existed and when knowledge industries were less prominent and technologically advanced. It has been difficult to update the agreement to reflect, for example recent advances in biotechnology, due to the consensus nature of the WTO, where certain Members routinely oppose reform. It is unsurprising that innovation-focused countries increasingly look to other avenues to ensure IP rules reflect modern realities, such as bilateral and regional trade agreements.
Nevertheless, TRIPS’ basic global floor on IP standards has underpinned the technological advancement and developmental progress of the last three decades, a process that has accelerated since the signature of the Doha Declaration in 2001. By setting the precedent that the TRIPS agreement can be disregarded, the proposed TRIPS waiver would spell the end of the agreement as a meaningful framework for IP protection. This would be a huge own goal.
