by Mark F. Schultz[1]
Key Takeaways
- Trade secrets are an increasingly important form of intellectual property that secure proprietary information. They serve as foundation for investment in innovation and encourage greater collaboration between businesses and other institutions by creating a foundation of trust.
- Trade secrets along with other IP rights helped enable the innovation, investment, and cooperation that has made possible the development and delivery of Covid-19 vaccines in record time.
- Many proponents of a proposal at the World Trade Organization to allow members to ignore or negate innovators’ intellectual property rights (IPRs) related to Covid-19 (i.e., ‘TRIPS Waiver’) misunderstand the function of trade secrets. Some have accused innovators of prolonging the pandemic by not surrendering trade secrets. As we explain here, trade secrets have enabled greater and faster technology transfer by establishing trust among partners.
- We document that innovators already are sharing secrets and know-how widely with dozens of partners across the world to produce vaccine and therapeutic doses as quickly as possible. In several instances, they are working closely with their biggest competitors, thanks to the security provided by trade secrecy and other IP laws.
- Forcing the disclosure of trade secrets would get in the way of manufacturing badly needed doses of Covid-19 vaccines by undermining voluntary arrangements and diverting resources from where they are needed most.
The biopharma industry has been praised for the unprecedented speed at which it developed Covid-19 vaccines and treatments, but the regard has not been universal. Before innovators had even finished clinical testing of vaccines in the autumn of 2020, India and South Africa proposed that the World Trade Organization allow members to disregard the Agreement on Trade-Related Aspects of Intellectual Property (TRIPS) so that they could ignore or negate innovators’ intellectual property rights (IPRs) related to Covid-19 (i.e., ‘TRIPS Waiver’). As vaccine manufacturing has ramped up, criticism of biopharma companies and their reliance on intellectual property rights has further increased. So has support for the proposed TRIPS Waiver, which proponents believe will increase global supply by allowing more companies to manufacture.
Implementing such an IP waiver throws up many practical challenges, not least the problem of trade secrets, a key IP right that protects the complex manufacturing processes that underpin the production of Covid-19 vaccines. Without access to these trade secrets, rival manufacturers cannot make high-quality generic versions of proprietary vaccines, as they will lack access to crucial formulas or “recipes” for key components of Covid-19 vaccines as well as manufacturing methods and know-how.
Given the importance of trade secrets to vaccine manufacturing, critics have accused innovators of prolonging the pandemic by not actively sharing know-how and preventing other manufacturers from ramping up production. One prominent critic contended that “the knowledge that can help end the pandemic should not be a secret.”[2] Some have urged that innovators be forced to divulge their trade secrets and know-how to speed up manufacturing.
This policy brief explains how trade secrets in fact play a fundamental and positive role in vaccine manufacturing and R&D.
First, trade secrets are not secrets, at least not in the way that people usually understand that term. Secrets are usually closely held and known by few. By contrast, trade secrets are a form of legal protection for commercially valuable proprietary information. To be a trade secret, information cannot be generally known in an industry and must be reasonably protected by the owner. However, the owner of a trade secret often shares it widely within a company and even outside a company, with proper precautions.
Second, if a trade secret owner sharing proprietary information outside of a company seems surprising, that surprise derives from another misunderstanding. So long as a trade secret owner protects the secret and shares it only selectively, not generally, it may remain a trade secret. The legal protection is both the enabler and key motivator of this sharing. Trade secret law makes it safer to collaborate with others, as legal protection encourages greater trust through greater security.
“Trade secret protection has helped enable the innovation, investment, and cooperation that has made possible the development and delivery of Covid-19 vaccines in record time.”
Third, and perhaps most important, trade secret protection has helped enable the innovation, investment, and cooperation that has made possible the development and delivery of Covid-19 vaccines in record time. In fact, the WTO TRIPS Waiver discussion overlooks an important fact. Innovators already are sharing secrets and know-how widely with dozens of partners across the world to produce vaccine and therapeutic doses as quickly as possible. In several instances, they are working closely with their biggest competitors. This sharing relies on IP rights such as trade secrets, as it allows proprietary information to be shared safely with the knowledge that it cannot be wrongly exploited by rivals for unauthorized commercial advantage.
Fourth and finally, there is a misconception that the TRIPS Waiver would speed up vaccine production. Much of this technology is both new and cutting edge, so only a limited number of producers can speed up production in the near to medium term. Those producers are already collaborating. In fact, forcing companies to divulge trade secrets would disrupt these partnerships, potentially leading to the disintegration of existing manufacturing supply chains. These partnerships are delivering on a scale that would have been unimaginable pre-pandemic, with global production of Covid-19 vaccines expected to reach more than 12 billion doses in 2021, according to Duke’s Global Health Innovation Center.[3]
This policy brief explores the importance of trade secrets to the response to the Covid-19 pandemic. The first section explains the nature of trade secrets – a legal right that protects proprietary information, and by doing so encourages greater openness, cooperation, and investment in innovation. The second section explains how IP rights, particularly trade secrets, have been an essential foundation to the fight against Covid-19. The third section explains how the proposed TRIPS Waiver would likely not provide the help intended and might cause harm by undermining trade secret protection.
1. Understanding Trade Secrets
 Many proponents of the WTO TRIPS Waiver appear to view trade secrecy as something that enables businesses to lock away knowledge solely for their own benefit, to the public’s detriment. This understanding seems to be motivated by instincts that transparency is better than secrecy and that people keep secrets for self-serving reasons.
Many proponents of the WTO TRIPS Waiver appear to view trade secrecy as something that enables businesses to lock away knowledge solely for their own benefit, to the public’s detriment. This understanding seems to be motivated by instincts that transparency is better than secrecy and that people keep secrets for self-serving reasons.
The problem with this view is that trade secrets are not secrets, at least as that term is conventionally understood.
Trade secret laws are an essential but often misunderstood innovation policy. Laws protecting trade secrets do not lock away information, but rather create a circle of trust within which businesses can use and share confidential information securely. The security of this legal protection encourages investment in innovation. Somewhat paradoxically, it also encourages greater cooperation and sharing of information – a factor that has proved highly relevant to Covid-19 vaccine manufacturing scale-up.
A. Defining Trade Secrecy
Trade secret laws protect confidential business information from being used or disclosed without permission. A trade secret is information that is neither generally known outside a business nor easy to uncover. It must have commercial value to the business, which gets an advantage from keeping it secret.
The commercial nature of trade secrets is key to understanding the purpose and nature of this legal protection. This legal protection is not meant to encourage hoarding of valuable information, but rather to create conditions where people are willing to create and use it to do business.
Thus, trade secret law will allow a business to share confidential information with its employees and even with other businesses, so long as it makes reasonable efforts to protect it. For example, it must have sound security on its computer systems and restrict access to its valuable information to those who need to know it. When it reveals trade secrets to employees or cooperating businesses, it usually needs to have them sign agreements to keep them secret.
Because trade secrets can be shared under protected conditions, they are not truly “secrets” as defined by common experience and understanding. A “secret” is usually something known to only a very few people. As the American statesman Benjamin Franklin famously said, “Three may keep a secret, if two of them are dead.” By contrast, a trade secret can be shared with many. It must be carefully guarded, but so long as it is shared under conditions of confidence and does not become generally known, trade secret law will prevent it from being disclosed or used without permission.
Trade secret protection is relatively fragile. In contrast to patent rights, trade secret rights do not prevent competitors from using any information that they independently discover or develop. So long as they do their own work rather than wrongly taking the work of others, trade secret protection does not apply. Competitors can also reverse engineer a trade secret from public information, including the product itself. If the trade secret becomes generally known, it ceases to be protected, which often happens because of leaks, publication by independent researchers such as academics, or because of the general advance of knowledge in an industry.

Many types of information can be a trade secret. There are two broad categories that are covered:
Technical information, for example:
- Research results and experimental data
- Product formulas and recipes
- Product designs
- Manufacturing processes
- Computer code
Business and financial information, for example:
- Customer lists
- Supplier lists
- Pricing information
- Marketing and business plans
B. Trade Secret Protection Is Key to R&D
Trade secret law importantly encourages innovation and investment by protecting the early stages of the R&D process. The European Commission explained this fundamental role of trade secrecy in its report proposing what eventually became the Trade Secret Directive (which harmonized trade secret laws among EU members):
Every IPR starts with a secret. Writers do not disclose the plot they are working on (a future copyright), car makers do not circulate the first sketches of a new model (a future design), companies do not reveal the preliminary results of their technological experiments (a future patent), companies hold on to the information relating to the launch of a new branded product (a future trademark), etc.[4]
Innovation is a process, and the entire IP system is essential to that whole process. Innovative developments – new products, methods, and processes – typically spring from a research and development process. This R&D process includes the development of a large amount of proprietary information and know-how developed through trial and error, learning what works and does not work, and deliberate testing.
This process and the information it generates may yield a patentable invention. If it does, however, that patented invention will represent the tip of an iceberg – the incremental, useful, and new invention that constitutes an inventive step over what came before. That “iceberg’s tip” will sit atop a large body of information, much of which the developer will depend on trade secret law to protect.
In a study for the Organisation for Economic Cooperation and Development prepared with co-author Douglas Lippoldt, we found a positive relationship between the effectiveness of countries’ trade secret protection and investment in innovation.[5] Stronger trade secret protection was associated with greater R&D spending, more researchers, more foreign direct investment, greater importance of high-tech services, and several other positive economic outcomes.[6]
C. Trade Secret Protection Secures Investment in Innovation
Creating the information embodied in R&D requires investment, and trade secret law secures those investments. Without trade secret protection, businesses would rightly fear that an unscrupulous employee or competitor might take its research and use it to unfairly compete. The unscrupulous competitor would gain a great advantage by skipping over the work and investment it takes to develop the product. Trade secret law provides a deterrent against and remedy for such wrongdoing.
“Although trade secrecy protects confidentiality, it paradoxically can lead to greater openness and collaboration.”
For all these reasons, businesses rely heavily on trade secrets. In fact, they may be the most important form of intellectual property, at least if you ask businesses. Numerous surveys in various countries with effective trade secret protection confirm the importance of trade secret protection. For example, a survey of European companies found that they preferred trade secrets to patents, with the preference strongest among smaller businesses.[7] A survey in the U.S. found similar results.[8] The European Commission sponsored a survey that illuminated the issue. In a survey of 537 businesses in Europe,[9] 75% of respondents ranked trade secrets as “strategically important to their company’s growth, competitiveness and innovative performance.” The survey found that companies of all sizes relied on trade secrets, including small and medium size enterprises.
D. Trade Secrecy Paradoxically Encourages Openness and Cooperation
Although trade secrecy protects confidentiality, it paradoxically can lead to greater openness and collaboration. A trade secret owner can rely at least partly on the law for protection, which brings “secrets” at least partly out from behind private, locked doors, making them safer to disclose and use for collaboration with others, including suppliers, manufacturers, and co-developers.
Effective trade secret laws lead to greater willingness to work with strangers. In economies where trade secret protection is ineffective, businesses are more inclined to remain family businesses or stay smaller.[10] Where businesses have the backing of the law to protect their secrets, they are more inclined to scale up by hiring new employees and add people with new and needed skills.
Even more important, trade secret law makes it safer to work with other businesses. Such collaborations are often essential – for example, another business might be better at manufacturing a product, fabricating a part, or providing information technology services. The relationship might even be a more formal one, such as a joint venture.
Such division of labour is the basis of modern economies; it is more efficient, it saves money, and it leads to growth and mutual benefit. However, if collaboration comes at the cost of endangering valuable secrets, it’s less likely to happen. Effective trade secret protection makes collaboration safer. There is always an element of confidentiality by design, but trade secrecy broadens the circle of trust.
A leading American trade secret law case, Rockwell Graphic Systems, Inc. v. DEV Industries,[11] illustrates how trade secrecy enables broader collaboration to the benefit of innovators, employees, collaborating businesses, and customers. Rockwell, a manufacturer of large printing presses, shared thousands of drawings and specifications with hundreds of employees and many contract manufacturers and customers to enable the quick manufacture of frequently needed replacement parts. Everyday understanding might not consider such widely shared information a secret. Nevertheless, it still received protection as a trade secret because the owner had maintained an unbroken circle of trust among its employees and business associates using security measures, including confidentiality agreements. Rockwell was thus able to serve its customers more effectively and affordably by sharing its information among its employees and working with a network of contract manufacturers.
Trade secrecy and other IP rights have, in fact, been fostering collaboration where it’s been most badly needed: In the effort to develop and manufacture treatments for Covid-19. We explain this in the next section.
2. Trade Secrets: Part of the Essential Foundation of the Fight against Covid-19
Producing Covid-19 vaccines and treatments has required continuing and significant innovation, investment, and collaboration at every step from lab to patient. The immediate drivers of this work were great science, skill, and dedication. But intellectual property, including trade secret protection, has been an essential foundation, creating the security, confidence, and trust that allows all this to happen.
There is a misunderstanding of the biopharma industry that causes some to underestimate the importance of IP to the industry and exactly why companies value their trade secrets. This view sees drug development as a straightforward undertaking, where a well-known, standard process is used to synthesize potential drug candidates, which are then tested to determine their potential, taken through clinical trials, and then if successful, mass manufactured using well-known, standard capabilities available widely around the world. In this view, the innovation is in identifying potential drugs and the risk and investment lies in taking them through clinical trials.
This view was never fully accurate and is increasingly outdated. Many drugs – particularly Covid-19 vaccines – are developed at great expense using cutting edge novel technology and are manufactured using novel, cutting edge techniques. This process requires innovation and investment at every step, relying on unique capabilities that are not necessarily widely available nor standard. Crucially, there has been ground-breaking innovation in both the original innovation and the manufacturing processes required to make that innovation into a mass-manufactured treatment for patients.
The reason for this change is that in recent decades, the biopharma industry has increasingly moved from its traditional focus on small molecule (chemical) drugs to large-molecule (biologic) drugs based on living systems.[12] Biologics have been successful in generating new treatments for hundreds of indications but are far more complex than small molecule drugs at every step, from development to regulatory approval to manufacturing.[13]
This shift to biologics has been reflected in Covid-19 vaccines and treatments – including mRNA vaccines (e.g., Pfizer/BioNTech and Moderna), adenovirus vector vaccines (e.g., J&J and AstraZeneca), protein subunit vaccines (e.g., Novavax), monoclonal antibodies (casirivimab and imdevimab), and antivirals (Remdesivir).
The increased complexity of developing and manufacturing treatments has made trade secrecy more important than ever to this industry. Trade secrecy is important to protecting the investment in innovation in new and diverse platform technologies and in developing manufacturing processes. As the industry has grown more complex, collaboration among companies, research institutions, manufacturers, and suppliers has become more necessary, with trust bolstered by trade secrecy.
For the purposes of this paper and related research, we interviewed intellectual property and manufacturing experts from eight major companies in the biopharma industry about the role of intellectual property in developing, manufacturing, and delivering Covid-19 vaccines and treatments.[14] Several common insights regarding the importance of trade secret protection emerged. The increasing complexity of developing and manufacturing means, as several interview subjects observed, that no one company can do it all alone. This has been particularly true in the case of developing Covid-19 vaccines and treatments, as companies moved as quickly as possible to address the challenge. Partnerships numbered in the hundreds, and often involved traditional rival companies working together to produce the needed vaccines and treatments.
As Matthew Pugmire, Assistant General Counsel at Pfizer, Inc., explained: “No one party can do everything. No one entity has all the tech to bring to bear to solve a problem like Covid. It has taken a tremendous amount of collaboration. And those IP aspects have really facilitated collaboration. It allowed parties to share information freely, knowing there are frameworks to protect that information so that it is properly used.”
In this Section, we explain how trade secrets have underpinned the investment, research collaboration, and innovation that has occurred at every step, from developing platform technology before the pandemic, to developing and manufacturing Covid-19 treatments and vaccines.
A. Developing Platform Technologies
In contrast to small molecule drugs, more recent innovation in the life sciences is characterised by an increasing number of platform technologies. A 2015 study counted at least “at least twenty innovative technologies” “used to create biotechnology products,” including recombinant DNA, molecular engineering of proteins, and monoclonal antibodies.[15] New technologies such as mRNA vaccines have arisen since.
Building on a new platform technology can constitute an “extra step” compared to developing small molecule drugs. While older drugs are developed based on more mature technologies, developing a new biologic may first require the development of new technologies and manufacturing techniques. Such R&D entails experimentation, adaptation, and creation of large amounts of data, an investment secured in large part by trade secret protection.
For Covid-19, new platform technologies have proven to be particularly significant. The first mRNA vaccines approved for use were the Moderna and Pfizer/BioNTech Covid-19 vaccines. The vaccines developed by Janssen/Johnson & Johnson and AstraZeneca used an adenovirus as a viral vector. The Novavax vaccine, which is still in development but shows promise, is a protein subunit vaccine produced with a novel manufacturing process using a living organism.
In our interviews, subjects observed that the technologies used to develop and manufacture such vaccines and treatments have value and application beyond Covid-19. For example, the mRNA technology used by Moderna and Pfizer/BioNTech has potential applications to treat cancer, multiple sclerosis and other conditions.[16]
The investment used to develop these technologies and resulting value is embodied in the R&D, the experiments, data, techniques, and know-how on which they are based. Our interview subjects observed that much of the value of the new vaccines and treatments developed by innovator companies lies in this information, but only if intellectual property rights – particularly patents and trade secrets – are protected.
The development of these platform technologies represented decades of IP-driven innovation and investment, some of which, like mRNA, bore fruit just in time to help with the pandemic. As
Pfizer’s Pugmire observed, “The core technologies came together at the right time and were available for the Covid response because we had a strong and robust IP system over the years. You could argue that those technologies would never have been developed without IP.”
For example, mRNA vaccines required key breakthroughs before they became practical as even potential vaccines.[17] These breakthroughs took decades of academic research to achieve. The patents on this academic research were licensed (and further sublicensed) to startups and other companies.[18] It then took about another ten years of applied R&D and billions in investment at companies such as BioNTech and Moderna to achieve viable treatments – which were fortunately ready by early 2020.[19]
The investments these companies obtained to support their applied work depended in part on their opportunity to get patents and protect trade secrets. As Moderna’s academic founder Derrick Rossi explained, “you can be working on the coolest thing, but investors need to know that there is some protection for their investment, plain and simple.” [20] IP is “the future prospect that reassures investors.” [21]
B. Developing Covid-19 Treatments
“No one party can do everything. No one entity has all the tech to bring to bear to solve a problem like Covid. It has taken a tremendous amount of collaboration. And those IP aspects have really facilitated collaboration. It allowed parties to share information freely, knowing there are frameworks to protect that information so that it is properly used.”
Matthew Pugmire, Assistant General Counsel, Pfizer
In the case of developing new treatments or repurposing older ones to combat Covid-19, trade secret protection was essential to bringing companies together in the first place. When companies sought to determine if they could productively work together, they had to disclose information about their research. A fundamental problem in such situations is that without protection, sharing information to demonstrate its value paradoxically destroys its value.
Consistently, companies cited trade secret protection as a key assurance that allowed them to reveal and exchange information, particularly in the context of virtual meetings required by the pandemic where personal trust was harder to develop. As Arno Hartmann , Head of Pharmaceutical Patents for Merck Group observed, “trade secret protection is an important factor. The first time you get in touch, you have to get a sense of the people on the other side. There is a certain level of trust needed. There is a series of engagements needed over time to build trust. People have to feel comfortable sharing and knowing they will get something in return. You build on this. Feeling that one can trust the other party is very important.”[23]
One of the most notable partnerships was between Pfizer and BioNTech. BioNTech’s founders, Ugur Sahin and Özlem Türeci, have been working on developing mRNA technology for the last 25 years and founded BioNTech in 2008.[24] BioNTech has a large range of collaborations with different pharmaceutical companies and organisations globally that hold patents for technology and research relating to mRNA, allowing BioNTech to then have a license to use the mRNA-based technologies to help them further the pioneering research in the field.[25]
When the pandemic arose, BioNTech decided to attempt to develop an mRNA vaccine.[26] It turned to Pfizer, already a partner on potential flu vaccines, because Pfizer had the manufacturing expertise and capacity to manufacture the vaccine at scale. They commenced work before a contract was signed, highlighting the trust within their relationship and ability to rely on trade secret protection.[27] They entered an agreement on March 17, 2020, whereby BioNTech agreed to disclose its mRNA research to Pfizer.[28] In return, Pfizer brought its significant manufacturing and regulatory know-how to bear on getting the vaccine approved and manufacturing it at scale.[29]
The level of trust needed for this fast-moving and deeply entwined collaboration was made feasible by trade secret protection. Without the security of trade secrecy, the risk might have been impracticable, particularly for BioNTech as the smaller party with deep expertise in mRNA. As Pfizer’s Pugmire observed, “IP protection was critical. We had an ongoing collaboration with BioNTech before the pandemic. . . . I can’t speak for them, but I cannot imagine they would be comfortable coming and sharing their mRNA construct with a company like Pfizer without IP protection. This is their core technology and the result of all the investments they have made over the years. IP protection gave them [the] assurance [that] they could share it without losing all their investments from over the years.”[30]
“Trade secret protection is an important factor. The first time you get in touch, you have to get a sense of the people on the other side. People have to feel comfortable sharing and knowing they will get something in return. You build on this. Feeling that one can trust the other party is very important”Arno Hartmann , Head of Pharmaceutical Patents, Merck Group
C. Manufacturing Covid-19 Treatments and IP
The biopharmaceutical industry’s shift to biologics has required greater innovation, investment, and cooperation among companies. Modern biopharma manufacturing is highly specialised, with manufacturing, finishing, and distribution distributed among many cooperating companies. The pandemic magnified the need for cooperation and specialisation in manufacturing.
The greater complexity in manufacturing biologics stems from the fact that biologic drugs are harvested from living microorganisms or produced from biologic processes, unlike small molecule drugs, which are synthesised using long established methods.[31] Rather than using a well-known process, innovators often must develop a new manufacturing process for the new drug and they likely need to work with others and license third party IP to do so.[32] A notable example of this novelty and complexity is the manufacturing process for the Novavax vaccine, which is produced with a novel manufacturing process using the cells of an armyworm moth larva as a living factory. While that example is striking in its particulars, it is like other biologics in that it employs a novel manufacturing process that requires multiple steps.
While a novel manufacturing process may quickly become standard, the innovation and investment in manufacturing Covid-19 vaccines is recent and continuing. Thus, on the one hand, not every biologic requires an entirely new manufacturing process, nor is every step in a new process novel. On the other hand, these manufacturing processes were made possible by recent, large investments of money and resources by innovators and investors. Without the security afforded by IP rights (and the expectation that these IP rights will continue to be enforced and respected), such investments likely would be put toward more relatively secure endeavors.
We explain in the following discussion how investment, innovation and cooperation in manufacturing Covid-19 treatments was secured by IP rights. To manufacture a new vaccine, an innovator may need to develop a new process, obtain materials from another specialised manufacturer, license technology from others, and share trade secrets and know-how with manufacturing partners. At each of these steps, IP, including trade secret protection, is necessary to enabling the required investment and collaboration.
1. Innovation in Manufacturing Covid-19 Treatments
 Scaling up the manufacture of new Covid-19 treatments has been challenging. Companies faced an unprecedented challenge to find the capacity to make billions of doses in a short time. While this challenge was great for all, it was particularly difficult with respect to mRNA vaccines, which had only ever been produced in small batches for research and testing purposes.
Scaling up the manufacture of new Covid-19 treatments has been challenging. Companies faced an unprecedented challenge to find the capacity to make billions of doses in a short time. While this challenge was great for all, it was particularly difficult with respect to mRNA vaccines, which had only ever been produced in small batches for research and testing purposes.
A misconception has arisen regarding mRNA vaccine production, with some saying that producing these vaccines is faster, easier, or quicker to scale up than other technology.[33] This may result from conflating the process of designing an mRNA vaccine with the task of mass manufacturing the vaccines. It is true that mRNA vaccines can be designed relatively quickly, albeit using knowledge and processes that took decades of trial and error and billons in investments to achieve.[34] Moderna famously worked out the design of its vaccine over the course of two days and started human clinical trials fewer than 60 days later.[35] Meanwhile, developing the manufacturing process was neither easy nor trivial, and it demands specialised and sophisticated capabilities. Granted, mRNA vaccines are quicker to manufacture than some other technologies, but that manufacturing process did not yet exist at the start of 2020, it took months of investment and innovation to develop, and the process is still undergoing refinement.
Manufacturing mRNA vaccines for the first time in a very short timeframe required significant innovation and remains a technically challenging endeavor. The process proved to be technically challenging as Pfizer developed a 50,000 step process.[36] Pfizer identified and worked with 86 different suppliers.[37] The vaccine required 280 materials in total, 10 to 15 of which were novel to the mRNA vaccine.[38] Through unstinting effort and investment, Pfizer cut the initial production time in half, enabling it to deliver more doses more quickly
The production process for Pfizer’s vaccine needs to be completed from start to finish inside a hermetically-sealed system.[39] It took several months of working with partners to identify the optimal process for making this mRNA vaccine, even though BioNTech had been working with mRNA technology for 25 years, possessed considerable knowledge related to mRNA platforms, and had spent 10 years setting up the manufacturing processes that allowed for their Covid-19 vaccine production.[40] According to the company itself, the validation of a single production site can still take up to a year.[41]
Moderna also innovated to develop its manufacturing capacity, which relied even more heavily on partnerships. For example, it partnered with Gingko Bioworks, which helped Moderna optimise processes for producing raw materials by providing “bioengineering know-how and resources.”[42] Moderna also entered a strategic partnership with a Swiss company, Lonza Ltd., in May 2020, to develop sufficient manufacturing capacity to meet global needs.[43] While Moderna’s scale up was assisted by U.S. government funding, Lonza provided the necessary know-how to develop a manufacturing process that could meet the regulatory requirements of over 50 countries.[44] In exchange, Moderna engaged in technology transfer to enable Lonza to develop its mRNA manufacturing capacity.[45]
“Just transferring the knowledge of filling and capping vaccine vials typically takes about 18 months and involves 10 stages, each consisting of hundreds of steps during which dozens of things can go wrong.”
Meanwhile, investment, innovation, and collaboration in improving mRNA manufacturing has continued. For example, in August 2021, Aldevron and Gingko Bioworks announced the first fruits of a partnership to optimise production of mRNA vaccine components.[46] They have developed a process to make the production of vaccinia capping enzyme (VCE) over 10 times more efficient.[47] VCE is a key component in mRNA vaccines, as it convinces the human immune system not to attack the active ingredient. VCE production has been difficult, so the breakthrough will help increase the production of mRNA vaccines. Aldevron has exclusive rights to the protocol for this new method[48] – presumably a trade secret unless they can and do choose to file a patent.
The heavy investment, technology transfer, and collaboration that enabled mRNA vaccine developers and their partners to develop a new manufacturing process in just a few months depended on IP. Parties cannot share their trade secrets without the trust provided by legal protection, nor justify investing their resources and others’ money on such an endeavour without the security that IP rights provide for a return on that investment.
2. Specialisation
Modern biopharma manufacturing is specialised, with manufacturing, finishing, and distribution distributed among many cooperating companies. Some of the manufacturers provide key components to vaccine developers and manufacturers. Others specialise in manufacturing but can only do so with technology transfer.
One example of specialisation is the production of plasmid DNA, a component that is used to produce mRNA Covid-19 vaccines and many other treatments. A study published in late 2020 identified the production of plasma DNA as “the bottleneck of the genetic medicine revolution.”[49] In August 2020, one plasmid DNA manufacturer observed that making just 2 billion doses of Covid-19 vaccine would use all the capacity the world then had for making plasmid DNA.[50] Since then, companies in this business have invested heavily and quickly to expand capacity.[51]
Plasmid DNA manufacturing is a competitive business where each manufacturer develops proprietary know-how. While customers tend to view plasmid DNA as a commodity product, each plasmid DNA manufacturer seeks competitive advantage by developing its own unique, proprietary processes and relying on its unique know-how for regulatory compliance and quality control.[52] A recent study of the biotech sector that interviewed industry experts found that trade secrecy in the manufacturing of biologics spurred greater innovation and the development of knowledge. Interviewees “reported that trade secrets covering the original product helped to spur innovation and increase scientific knowledge. Lacking information on the development of the originator biologic, biosimilar companies are often forced to develop their own processes, resulting in improved understanding of the biologic active substance’s characteristics and function.”[53] Trade secrets help drive competition, and thus innovation and investment in this sector.
The demand for plasmid DNA and the limitations of the current manufacturing technology call for further innovation. The late 2020 study that identified the plasmid DNA bottleneck stated that “it is becoming increasingly clear that it is the fundamentals of plasmid DNA manufacture that render it incapable of enabling the future of genetic medicine” as the current process is too slow and prone to failure.[54]
In response to this need, Touchlight Genetics Ltd developed a proprietary process for producing a synthetic DNA vector. This patented technology, referred to as ‘doggybone DNA (dbDNA), is an alternative to plasmid DNA.[55] It can be produced in weeks rather than the months required for plasmid DNA, and it states that it can produce enough for 1 billion vaccine doses a month.[56] While the technology itself is patented, Touchlight has worked for several years to optimise its manufacturing techniques. In 2018, Touchlight partnered with Janssen Biotech to evaluate and refine its production processes for its patented technology.[57] These sorts of key collaborations, requiring technology transfer, as well as the development and sharing of valuable proprietary information, depend on the trust engendered by trade secret laws and other forms of IP protection.
3. Collaboration in Manufacturing
The demands of the pandemic made cooperation among specialised producers even more urgent. While large corporations like Pfizer and J&J do have some production capacity, their facilities cannot produce vaccine doses on the billions scale needed to vaccinate the world against Covid-19.
Given the enormity and urgency of the task, innovator companies could not and did not “hoard” vaccine trade secrets and know-how. To increase manufacturing capacity, they turned not only to contract manufacturers but to companies that were normally competitors. They had to teach these manufacturers how to make their products. Under these circumstances, trade secret laws and other IP protections accelerated rather than impeded vaccine manufacturing by enabling the necessary trust and confidence.
The Wall Street Journal recently reported on Pfizer’s search for manufacturing partners and work to transfer technology. The report described the work of Pfizer’s team, which is “among a relatively small number of professionals with the rare skill set to enable other companies to produce the shots.”[58] One team member’s full-time job is to find potential partners with the capability to implement mRNA manufacturing technology.[59]
As it began to look for manufacturing partners, Pfizer found that it was in a competitive situation. “Pfizer was vying with rival Covid-19 vaccine developers for limited manufacturing space . . . and it needed partners willing to work without a proven product.”[60] Partnering with Pfizer initially took a leap of faith: “Some of the would-be partners expressed frustration that [Pfizer] didn’t have more details about the Pfizer-BioNTech vaccine candidate because it was a work in progress.”[61]
Once Pfizer finds and secures a potential partner, it takes months of transfer of the disclosure of confidential information and training to get the partner up to speed. The Wall Street Journal report described the lengthy process of bringing Pfizer’s partner, Thermo Fisher, up to speed. In May 2020, Pfizer began discussions with Thermo Fisher. The parties spent several months exchanging information as Pfizer developed its vaccine and manufacturing processes. Once the parties anticipated an agreement, a 24-person team from Pfizer began transferring know-how even before a final agreement was reached in February 2021. Among other things, Pfizer disclosed “more than 500 top-secret files – at least 5,000 pages of documents on making the vaccine – over secure computer servers and trained Thermo Fisher workers on mRNA, which the plant had never used before.”[62] Seven months later in late August 2021, the parties are completing the process and submitting data to regulators for approval for Thermo Fisher to begin manufacturing.[63]
Once a vaccine’s components are manufactured by Pfizer or a partner such as Thermo Fisher, they need to be assembled and packaged into vaccine vials through a fill-and-finish process and then distributed. This work often involves a second set of specialist companies. For these arrangements, yet more know-how must be shared and transferred. The Wall Street Journal reported that “just transferring the knowledge of filling and capping the vials typically takes about 18 months and involves 10 stages, each consisting of hundreds of steps during which dozens of things can go wrong.”[64]
Without protection for the know-how and trade secrets of Pfizer and BioNTech, this collaboration
and transfer of know-how could not have occurred. With mRNA vaccines potentially the basis of many other future treatments, from Multiple Sclerosis to cancer, the technology needed to be secured before it could be shared. As Bryan Zielinski, Chief Patent Counsel at Pfizer observed, “the same way that BioNTech was able to work with Pfizer due to IP protection, we were able to work with partners on manufacturing deals. Patents provided security, in addition to know-how and trade secret protections.”[65]
All of this collaboration debunks the Covid-19 vaccine production story being told or implied by some critics. Some of the stronger rhetoric might lead one to believe that a few innovator companies are holding up the supply of vaccines by “going it alone” in order to jealously guard their know-how and secrets.
This story is simply not true. Instead, innovators are all collaborating with a wide variety of partners – suppliers, manufacturing partners, fill and finish providers, and distributors. They are necessarily sharing trade secrets and know-how to make this happen. The key limiting factors are finding partners with the necessary skills and equipment to do the work and meet stringent regulatory requirements.
Makers of Covid-19 therapeutics also engaged in rapid and extensive technology transfer to manufacturing partners. Gilead Sciences licensed generic pharmaceutical manufacturers in Egypt, India, and Pakistan to manufacture its broad spectrum antiviral Remdesivir.[66] The contracts included technology transfer, as explained by Hemal Shah, Director of International IP & Trade Policy at Gilead, “Ultimately we share our IP in order to show our partners how to safely and effectively make the product. Onboarding manufacturers involves significant technology transfer and you need to be able to share and speak freely.”[67] Shah also observed that voluntary, collaborative technology transfer is not a one time, one way event, as both Gilead and its partners have discovered and shared with one another improvements and refinements in production processes.
One way to show the scope of collaboration is to summarise it. Collaborations are not centrally reported but many are publicly announced, and we have collected this information as available from a wide variety of sources.
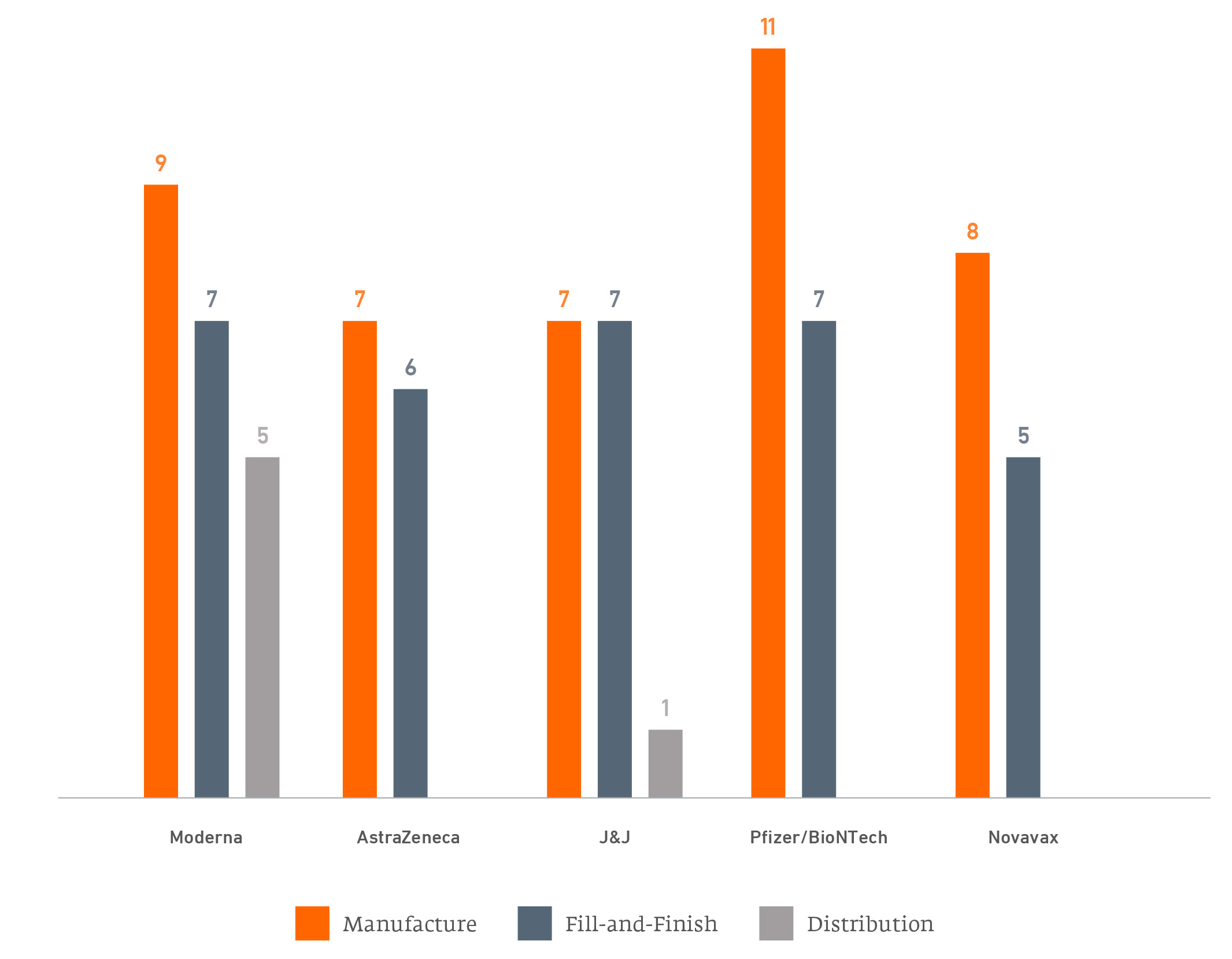
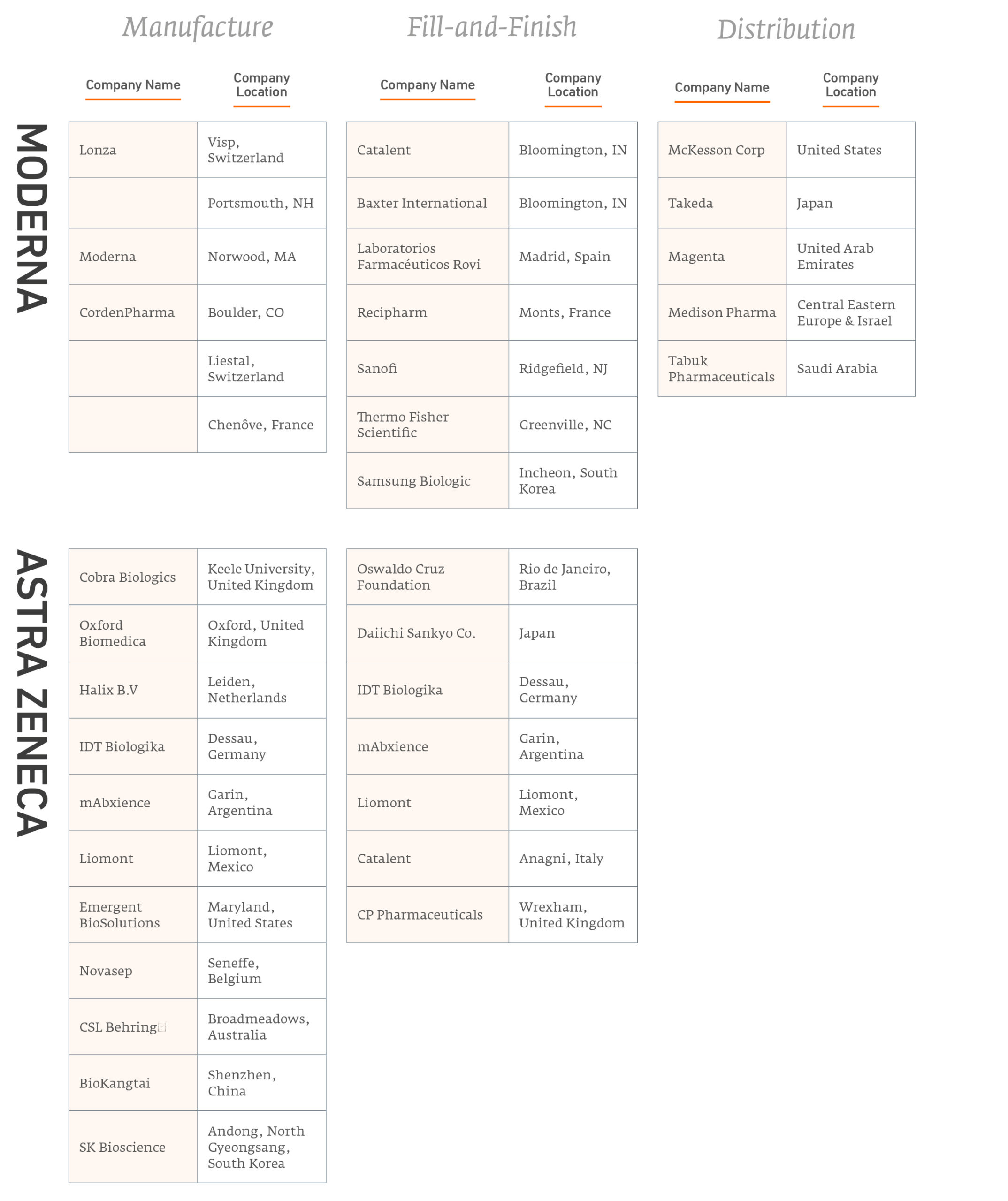
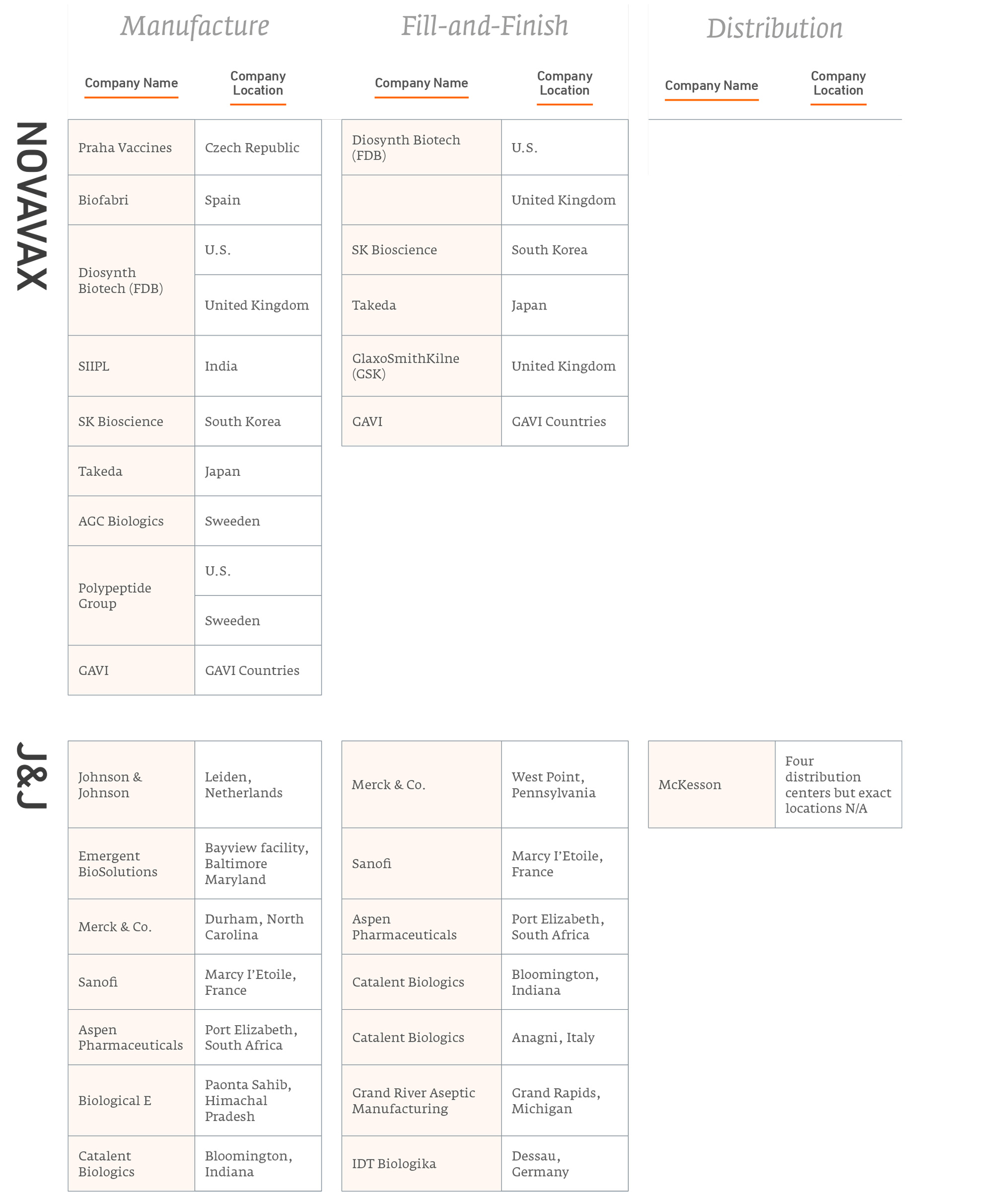
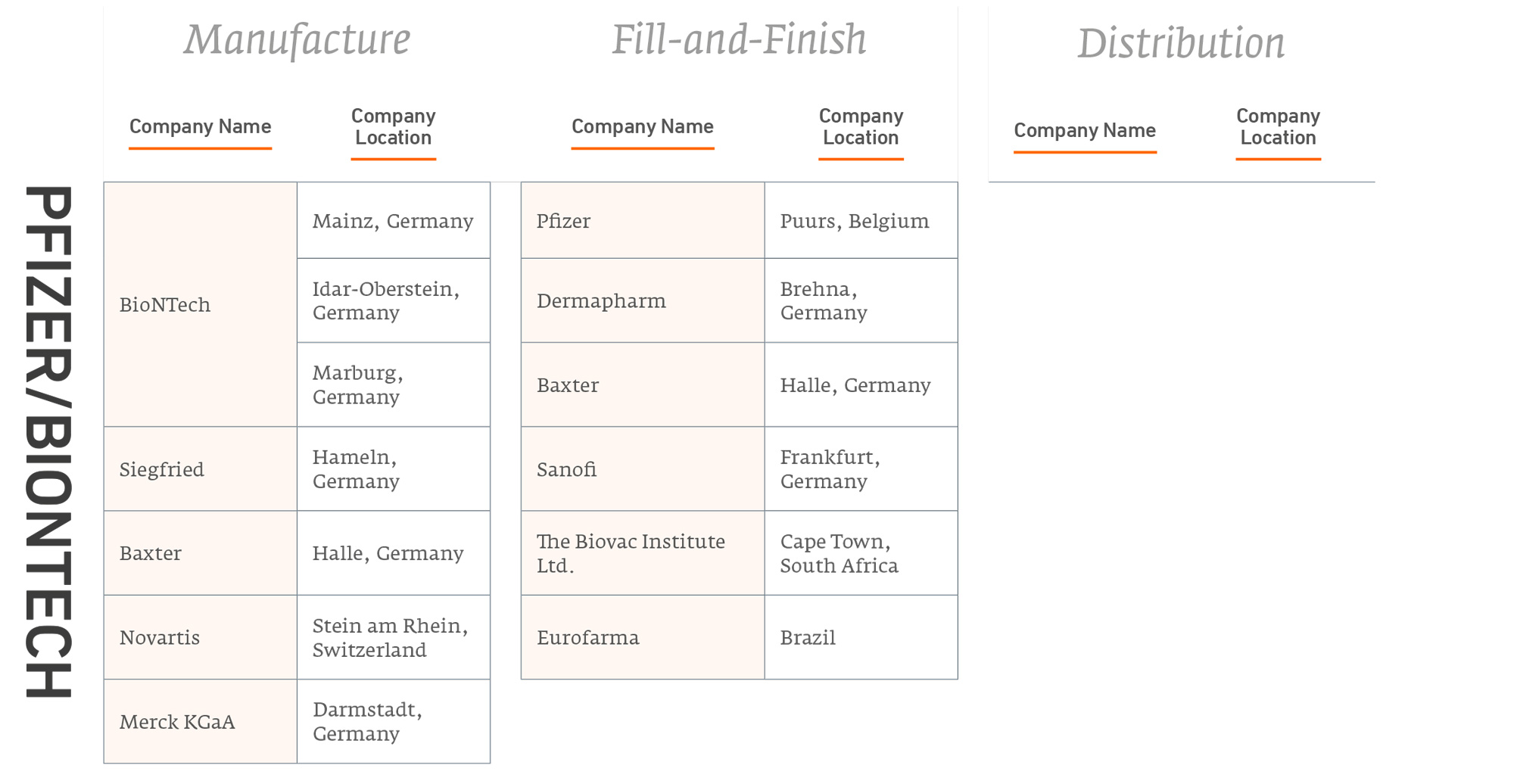
Figure 1 summarises publicly-announced manufacturing and distribution partnerships from each of the five leading vaccine companies innovators.
Number of Agreements for Vaccine Production
Figure 2 summarises the partnerships these innovators have entered to manufacture their products. Note, some of these partnerships are with major competitors.
Covid-19 vaccine manufacturing partnerships
3. Why the TRIPS Waiver is Likely to be Counterproductive

Despite the huge investment and unprecedented collaboration to ramp up production of Covid-19 vaccines, critics say it is not enough. Calls have been increasing to implement the proposed TRIPS Waiver at the WTO. This waiver would allow member nations to pass legislation or otherwise disregard obligations they have under the WTO Agreement on Trade Related Aspects of Intellectual Property (TRIPS). The proposed wavier would apply to patents, copyrights, trade secrets, and designs.
Some are further proposing that makers of Covid-19 vaccines and treatments be required to surrender know-how pursuant to the proposed waiver. But such a coerced transfer of know-how poses several problems and would prove counterproductive both in the long and short run. It would destroy valuable trade secrets, thus reducing incentives to address future emergencies. Just as important, it would interfere with addressing the current emergency as it would, paradoxically, reduce cooperation and information sharing.
Here are some of the key issues with the proposed TRIPS Waiver:
Trade Secrets Are Not the Problem. Critics are portraying innovators and IP laws as the reason vaccine production is slower than one would like. While it is tempting to look for a villain when things aren’t going as well as one would hope, the facts do not support this story.
While there are key trade secrets underlying Covid-19 production, nobody is hoarding information. As this paper explains, this statement is not the paradox it seems to be. Trade secret laws allow people to rely on legal protection instead of zealously guarding information within a small circle. Instead, information can be revealed to more employees and third-party businesses without losing proprietary status.
In this way, trade secrets and other forms of IP enable greater sharing of information, which is exactly what has happened in the case of Covid-19. As we document, innovators are collaborating widely with third parties, engaging in transfer of proprietary information and know-how, even to competitors.
The holdup is in part caused by the fact that this is a new technology, requiring sophisticated manufacturing capabilities. Some of the components, such as plasmid DNA, were not yet being produced in the needed quantities the pandemic response required.
Moreover, innovators do not appear to be holding back as they transfer technology and know-how to maximise production. In interviews, they consistently said that they are voluntarily transferring know-how to every manufacturer that can employ these complex technologies quickly and safely. The fact that this knowledge transfer is so widespread and includes competitors is confirmation of this assertion, as is the Wall Street Journal account of Pfizer’s willingness to share thousands of pages of confidential documents and engage in months of training of a manufacturing partner.
“A trade secret revealed in any one country would cease to exist everywhere. This total loss contrasts with what happens when a patent is compulsorily licensed. Such a forced disclosure could result in the largest peacetime destruction of the value of intellectual property rights in history.”
A Trade Secret Revealed Without Protection is a Trade Secret Destroyed. The problem with forcing disclosure of confidential information is that once revealed, its commercial value is destroyed. A trade secret revealed in any one country would cease to exist everywhere. This total loss contrasts with what happens when a patent is compulsorily licensed. While it may be less valuable in one country, it still retains value in other countries and for other uses. The difference in loss of value is like the difference between requiring the free rental of a single room in a skyscraper and demolishing the entire building.
Such a forced disclosure could result in the largest peacetime destruction of the value of intellectual property rights in history. Given the fact that many of these trade secrets relate not just to Covid-19 vaccines and treatments but are applicable to cutting edge technologies more generally, the losses could be many billions of dollars. Depending on the laws of the country forcing disclosure, the loss may even go uncompensated and create a disincentive to continue R&D.
Widespread Collaboration and Specialization Means Knowledge and IP Rights are Widely Distributed. In the modern biopharma industry, no single innovator has all the rights, knowledge and skills that are needed to enable third parties to develop and manufacture vaccines. In the first place, all the innovators have licensed in key technology developed in basic research institutions. More important, they rely on a variety of partners with their own expertise to provide key components. While these obstacles are not insurmountable, they make the challenge more complex than “making Astra Zeneca or Moderna give up their secrets.” For example, the plasmid DNA producers discussed earlier are a key part of production.
Involuntary Sharing of Know-how is Impracticable. Reading a patent or other disclosure can teach a technically trained person a great deal but manufacturing sophisticated biopharma products that must meet exacting regulatory standards takes hands-on experience. For this reason, many proponents of the TRIPS Waiver are advocating for the transfer of know-how.
“Only some manufacturing know-how can be written in a manual. Most must be learned on the job. Reluctant teaching is unlikely to produce effective transfer.”
“Know-how” is an imperfectly defined category of information, but the name itself is broadly indicative – it’s the knowledge of how to do something. It may include trade secrets, but it is a much broader category. Companies and individuals acquire skills and knowledge that aren’t necessarily proprietary or unique to a particular business, but that require experience to develop. Real life conditions generate all sorts of problems and failures that one learns to overcome. For example, one manufacturer told us that the extremely cold temperature that mRNA vaccines require make it hard to affix the necessary label to vials. Experience in overcoming such problems – including far more technical challenges – generates know-how.
As Novartis’s Salsberg observed, “people don’t usually set out to develop know-how. Rather, it is often the natural product of doing scientific and technical work. It’s hard to distil and put in a manual. Real know-how cannot just be written on a paper. You have to share know-how through doing and through collaboration.”[68]
The need for hands on collaboration is the flaw underlying proposals to require or strongly persuade companies to transfer know-how. Only some know-how can be written in a manual. Most must be learned on the job. Reluctant teaching is unlikely to produce effective transfer.
Forced or reluctant technology transfer also would be a distraction from fighting the pandemic. Because not all know-how is reducible to a written description, people are needed to collaborate. Effective transfer of know-how would require redirecting key individuals and business units away from the mission of fighting the pandemic or concentrating on the most technically capable voluntary partners first. The forced relationship would lack the beneficial two-way, ongoing communication of voluntary relationships.
What is more likely to produce effective know-how transfer and speed production is voluntary collaboration for mutual gain. That is already happening around the world.
Taking Trade Secrets Will Likely Chill Innovation and Collaboration. The TRIPS Waiver likely won’t make the largest biopharma companies stop innovating or even make them completely unwilling to step up for the next crisis. But it could reduce investment in new companies and reduce resources available to existing ones by dissuading investors from this sector. It could also change the way in which biopharma companies are willing to help during a crisis.
“What is more likely to produce effective know-how transfer and speed production is voluntary collaboration for mutual gain. That is already happening around the world.”
A large-scale loss of trade secrets would chill investment by adding an additional risk for investors to consider. Successful companies such as Moderna and BioNTech raised billions of dollars from investors when they were startups. So did many less well-known unsuccessful companies, as most biopharma startups fail. Investors must accept the likelihood of failure when they invest. However, if they also must account for the fact that success also creates a political risk of loss, then investments in less politically risky startups in fields such as fashion or food and beverages become comparatively more attractive.[69] Increasing risk would not end all investment, but more risk leads to fewer investments and less money invested. Reducing investment in the biopharma sector would mean fewer future Modernas and BioNTechs.
Moreover, the TRIPS Waiver could teach a harsh and counterproductive lesson that volunteering to apply technologies to global health problems will result in loss. Companies may be inclined to limit the potential exposure of trade secrets by pulling back from relationships and countries where they perceive the risk of expropriation to be high. This would result in less collaboration and less manufacturing in middle income countries and other jurisdictions perceived to be risker.
In addition, some of the technology involved here – for example, mRNA vaccines – is among the most promising for the industry. Forced disclosure would give global competitors an edge at the expense of innovators who had invested the resources to develop these technologies. Governments concerned with national competitiveness would do well to consider the implications.
4. Conclusion
Trade secrets and other IP rights have played a positive role in enabling the innovation, investment, and collaboration that has delivered Covid-19 vaccines in record time. They continue to enable mass manufacturing of vaccines at unprecedented levels by enabling collaboration, technology transfer, and continuing innovation.
As we have documented, no innovator is going it alone, jealously refusing to share their knowledge. They are widely collaborating, working willingly with partners who are able to quickly meet the challenge of manufacturing sophisticated, novel products. The fact that these partnerships include competitors and require extensive technology transfer is telling.
The TRIPS Waiver proposal would undermine innovation and collaboration, now and in the future. Its unprecedented proposal to allow countries to destroy trade secrets and require involuntary transfer of know-how would distract innovators from addressing this pandemic and chill future collaboration, investment, and innovation.
The cost would be high, with little benefit. The producers with capacity to produce these novel vaccines (not just simple medicines or traditional vaccines) are already deployed. The private sector has spent large sums to address production bottlenecks, such as increasing the supply of plasmid DNA.
While governments may consider future capacity, they would do best to consider how they could share and distribute the doses of vaccine they control with those around the world at the highest risk.
[1] Professor & Goodyear Endowed Chair in Intellectual Property Law, University of Akron, USA. Thanks to Geneva Network for financial support in the research and preparation of this article. Geneva Network has received funding from a range of public sector, non-governmental and private sector organizations (including the biopharma industry). The views and conclusions expressed herein are entirely those of the author.
[2] Dan Diamond & Yasmeen Abutaleb, “‘Act now’ on global vaccines to stop more-dangerous variants, experts warn Biden,” Washington Post, August 10, 2021, https://www.washingtonpost.com/health/2021/08/10/health-experts-demand-global-vaccines-pandemic/
[3] Andrea Taylor et al., Issue Brief: Deciphering the Manufacturing Landscape for Covid-19 Vaccines, Duke Global Health Innovation Center, March 2021, https://launchandscalefaster.org/sites/default/files/documents/Speedometer%20Issue%20Brief-COVID%20Manufacturing%20Landscape%2019%20March%2021.pdf
[4] Baker and McKenzie (2013), Study on Trade Secrets and Confidential Business Information in the Internal Market, prepared for the European Commission (EC), p. 2
[5] Lippoldt, D. C. and Schultz, M. F. 2014. “Uncovering Trade Secrets – An Empirical Assessment of Economic Implications of Protection for Undisclosed Data.” OECD Trade Policy Papers, No. 167, OECD Publishing, doi: 10.1787/5jxzl5w3j3s6-en
[6] Ibid.
[7] Arundel, A. (2001), “The Relative Effectiveness of Patents and Secrecy for Appropriation”, 30 Research Policy, 611–624.
[8] Cohen, W., R. Nelson and J. Walsh (2000), “Protecting their Intellectual Assets: Appropriability Conditions and Why U.S. Manufacturing Firms Patent (Or Not)”, NBER Working Paper, W7552, http://www.nber.org/papers/w7552.pdf
[9] Baker and McKenzie (2013), Study on Trade Secrets and Confidential Business Information in the Internal Market, prepared for the European Commission (EC),
[10] Rajbhandary, A. (1996), “Protecting Trade Secrets Through Family Businesses: A Case Study On Nepal,” International Review of Law and Economics, 16.4, 483-490; Sherwood, R.M. (1990), Intellectual Property and Economic Development, Westview Special Studies in Science, Technology, and Public Policy.
[11] 925 F.2d 174 (1991).
[12] Evens, R. & Kaitin, K. Health Aff. 34, 210–219 (2015).
[13] Ibid; Evens, R. P. AAPS J. 18, 281–285 (2016).
[14] The parallel project is forthcoming, “Unprecedented: The Rapid Innovation Response to COVID-19 and the Role of IP,” Innovation Council (Forthcoming 2021).
[15] Evens, R. & Kaitin, K. Health Aff. 34, 210–219 (2015).
[16] Stacey Colino, New cancer treatments may be on the horizon—thanks to mRNA vaccines, National Geographic, July 8, 2021, available at https://www.nationalgeographic.com/science/article/new-cancer-treatments-may-be-on-the-horizonthanks-to-mrna-vaccines
[17] Garde, Damian and Jonathan Saltzman. “The Story of MRNA: From a Loose Idea to a Tool That May Help Curb Covid.” STAT, November 10, 2020. https://www.statnews.com/2020/11/10/the-story-of-mrna-how-a-once-dismissed-idea-became-a-leading-technology-in-the-covid-vaccine-race/
[18] Ibid.
[19] Ibid.
[20] Mark Schultz, “The Importance of an Effective and Reliable Patent System to Investment in Critical Technologies,” USIJ, 2020.
[21] Mark Schultz, “The Importance of an Effective and Reliable Patent System to Investment in Critical Technologies,” USIJ, 2020.
[22] Interview conducted for this study and “Unprecedented: The Rapid Innovation Response to COVID-19 and the Role of IP,” Innovation Council (Forthcoming 2021).
[23] Ibid.
[24] Garde, Damian and Jonathan Saltzman. “The Story of MRNA: From a Loose Idea to a Tool That May Help Curb Covid.” STAT, November 10, 2020. https://www.statnews.com/2020/11/10/the-story-of-mrna-how-a-once-dismissed-idea-became-a-leading-technology-in-the-covid-vaccine-race/; Pancevski, Bojan, and Jared S. Hopkins. “How Pfizer Partner BioNTech Became a Leader in Coronavirus Vaccine Race.” Wall Street Journal, October 22, 2020, sec. Business. https://www.wsj.com/articles/how-pfizer-partner-biontech-became-a-leader-in-coronavirus-vaccine-race-11603359015
[25] Filiou, “The Pfizer-BioNTech Vaccine: Openness and Collaboration to Tackle the World’s Problems,” Open University UK, https://business-school.open.ac.uk/news/pfizer-biontech-vaccine-openness-and-collaboration-tackle-world’s-problems
[26] BioNTech. “BioNTech: Be Unique, Treat Individualized.” Accessed July 28, 2021. https://www.biontech.de.
[27] Pancevski and Hopkins, “How Pfizer Partner BioNTech Became a Leader in Coronavirus Vaccine Race.”
[28] Ibid
[29] Ibid
[30] Interview conducted for this study and “Unprecedented: The Rapid Innovation Response to COVID-19 and the Role of IP,” Innovation Council (Forthcoming 2021).
[31] Evens and Kaitin.
[32] Vulto, Jaquez, “The process defines the product: what really matters in biosimilar design and production?,” Rheumatology, 8-2017; Louise C. Druedahl et al, “A Qualitative Study of Biosimilar Manufacturer and Regulator Perceptions on Intellectual Property and Abbreviated Approval Pathways,” Nature Biotechnology, 38.11 (2020), 1253–56 <https://doi.org/10.1038/s41587-020-0717-7>;.
[33] Christopher Morten et al., “Pharma’s secrecy hinders global COVID-19 vaccination. Joe Biden could fix that.” Boston Globe, June 1, 2021.
[34] Garde, Damian and Jonathan Saltzman. “The Story of MRNA: From a Loose Idea to a Tool That May Help Curb Covid.” STAT, November 10, 2020. https://www.statnews.com/2020/11/10/the-story-of-mrna-how-a-once-dismissed-idea-became-a-leading-technology-in-the-covid-vaccine-race/
[35] Clifford, “How the Moderna Covid-19 mRNA vaccine was made so quickly,” CNBC, July 9, 2021, https://www.cnbc.com/2021/07/03/how-moderna-made-its-mrna-covid-vaccine-so-quickly-noubar-afeyan.html
[36] Park, Alice, and Aryn Baker. “Exclusive: Inside the Facilities Making the World’s Most Prevalent COVID-19 Vaccine.” Time, April 19, 2021. https://time.com/5955247/inside-biontech-vaccine-facility/. Neubert and Scheitz, “Exploring the Supply Chain of the Pfizer/BioNTech and Moderna COVID-19 Vaccines.”
[37] Interview conducted with Pfizer for this study and “Unprecedented: The Rapid Innovation Response to COVID-19 and the Role of IP,” Innovation Council (Forthcoming 2021).
[38] Ibid.
[39] Park and Baker, “Exclusive: Inside the Facilities Making the World’s Most Prevalent COVID-19 Vaccine.”
[40] Garde and Saltzman, “The Story of MRNA: How a Once Dismissed Idea Became a Leading in the Covid Vaccine Race.”; Pancevski and Hopkins, “How Pfizer Partner BioNTech Became a Leader in Coronavirus Vaccine Race.”; BioNTech, “BioNTech.”; David, “Covid: The Vaccine Patent Row Explained.”
[41] BioNTech, “BioNTech.”; David, “Covid: The Vaccine Patent Row Explained.”
[42] “Ginkgo Bioworks Provides Support on Process Optimization to Moderna for COVID-19 Response,” BioSpace, April 15, 2020, https://www.biospace.com/article/releases/ginkgo-bioworks-provides-support-on-process-optimization-to-moderna-for-covid-19-response/
[43] “Moderna and Lonza Announce Worldwide Strategic Collaboration to Manufacture Moderna’s Vaccine (mRNA-1273) Against Novel Coronavirus”, Press Release, May 1, 2020, https://investors.modernatx.com/news-releases/news-release-details/moderna-and-lonza-announce-worldwide-strategic-collaboration
[44] See ibid.
[45] Ibid.
[46] Alex Keown, “Aldevron, Ginkgo Bioworks Tout “Breakthrough” in mRNA Manufacturing,” BioSpace, August 10, 2021, https://www.biospace.com/article/aldevron-ginkgo-bioworks-make-breakthrough-in-manufacturing-components-for-mrna-vaccines/
[47] Ibid.
[48] Ibid.
[49] Jonny Ohlson, “Plasmid manufacture is the bottleneck of the genetic medicine revolution.” Drug discovery today, vol. 25,11 1891–1893. 16 Oct. 2020, doi:10.1016/j.drudis.2020.09.040
[50] Vivienne Raper, Plasmid Manufacturer Scales Up Production Capacity to Serve New Therapeutics, GEN, August 11, 2020, https://www.genengnews.com/topics/bioprocessing/plasmid-manufacturer-scales-up-production-capacity-to-serve-new-therapeutics/
[51] Ibid.; Gareth Macdonald, “Thermo takes aim at growing plasmid DNA manufacturing market with new plant,” BioProcess International, July 20, 2021, https://bioprocessintl.com/bioprocess-insider/facilities-capacity/thermo-takes-aim-at-growing-plasmid-dna-manufacturing-market-with-new-plant/
[52] Phase-Dependent Approaches to Plasmid DNA Manufacture, GEN, August 2, 2021, https://www.genengnews.com/resources/tutorial/phase-dependent-approaches-to-plasmid-dna-manufacture/
[53] Druedahl, L.C., Almarsdóttir, A.B., Kälvemark Sporrong, S. et al. A qualitative study of biosimilar manufacturer and regulator perceptions on intellectual property and abbreviated approval pathways. Nat Biotechnol, 2020.
[54] Jonny Ohlson, “Plasmid manufacture is the bottleneck of the genetic medicine revolution.” Drug discovery today, vol. 25,11 1891–1893. 16 Oct. 2020, doi:10.1016/j.drudis.2020.09.040
[55] Dan Stanton, “With $58m in hand, Touchlight looks to triple DNA manufacturing capacity,” BioProcess International, March 18, 2021, https://bioprocessintl.com/bioprocess-insider/facilities-capacity/with-58m-in-hand-touchlight-looks-to-triple-dna-manufacturing-capacity/
[56] Ibid.
[57] “Touchlight announces collaboration with Janssen Biotech,” Press Release, October 24, 2018, https://www.touchlight.com/news/company-news/touchlight-announces-collaboration-with-janssen-biotech/
[58] Jared S. Hopkins, “Key Staffers Aid Global Vaccine Rollout,” Wall Street Journal, August 20, 2021, https://www.wsj.com/articles/pfizers-global-covid-19-vaccine-rollout-depends-on-two-expert-staffers-11629464010?page=1
[59] Ibid.
[60] Ibid.
[61] Ibid.
[62] Ibid.
[63] Ibid.
[64] Ibid.
[65] Interview conducted for this study and “Unprecedented: The Rapid Innovation Response to COVID-19 and the Role of IP,” Innovation Council (Forthcoming 2021).
[66] Gilead. “Voluntary Licensing Agreements for Remdesivir,” 2020. https://www.gilead.com/purpose/advancing-global-health/covid-19/voluntary-licensing-agreements-for-remdesivir
[67] Interview conducted for this study and “Unprecedented: The Rapid Innovation Response to COVID-19 and the Role of IP,” Innovation Council (Forthcoming 2021).
[68] Interview conducted for this study and “Unprecedented: The Rapid Innovation Response to COVID-19 and the Role of IP,” Innovation Council (Forthcoming 2021).
[69] Mark Schultz, “The Importance of an Effective and Reliable Patent System to Investment in Critical Technologies,” USIJ, 2020 (showing that U.S. venture capital investment has, in relative terms, declined in biopharma while increasing in food, beverage and fashion as U.S. patents have become more difficult to obtain and enforce).